Journal of Catalysis ( IF 6.5 ) Pub Date : 2021-12-03 , DOI: 10.1016/j.jcat.2021.11.037 Yulin Zhou 1 , Francois Bihl 1 , Antoine Bonnefont 1 , Corinne Boudon 1 , Laurent Ruhlmann 1 , Vasilica Badets 1
|
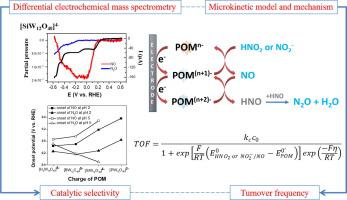
In this paper we have studied the influence of the charge and redox potential for a series of polyoxometalates (POMs) belonging to the Keggin family on the electrochemical reduction of nitrite or nitrous acid (NO2– or HNO2). We have focused on the correlation of gaseous products and the redox properties of the POMs. Analysis by differential electrochemical mass spectrometry (DEMS) showed that the gaseous products (NO and N2O) are formed at pH 2 and 5 by reaction of HNO2 or NO2– with the reduced POM. Only NO is detected when the POM ([PW12O40]3- , [SiW12O40]4-, [BW12O40]5-) undergoes the first one-electron reduction wave. However, N2O is obtained along with NO as soon as the POM undergoes two-electron reduction. The potential of the first reduction wave of the POM is correlated with its charge. Consequently NO and/or N2O appear at lower overpotential for [PW12O40]3- and [SiW12O40]4- followed by [BW12O40]5- and [H2W12O40]6-. Microkinetic simulations of the experimental current–potential curves were used to estimate the rate constant kc of the reaction between the reduced POM and HNO2 or NO2– in solution and the turnover frequency (TOF). At low overpotentials, the values of TOF are decreasing in a trend following the redox potential of the POM: [PW12O40]3- > [SiW12O40]4- > [BW12O40]5- while at high overpotentials the TOFs are dominated by kc following the reverse order. At pH 5 kc values are about 70 times lower than at pH 2. This is attributed to the decrease in the proton concentration and to the electrostatic repulsion between the negatively charged POMn- and NO2–.
中文翻译:

一系列 Keggin 多金属氧酸盐催化亚硝酸盐电还原的选择性和效率
在本文中,我们研究了一系列属于 Keggin 家族的多金属氧酸盐 (POM) 的电荷和氧化还原电位对亚硝酸盐或亚硝酸(NO 2 –或 HNO 2 )电化学还原的影响。我们专注于气态产物与 POM 氧化还原特性的相关性。(DEMS)显示分析通过差电化学质谱,所述气态产物(NO和N 2形成O)在通过HNO的反应pH 2和5 2或NO 2 -与所述降低的POM。当 POM ([PW 12 O 40 ] 3- , [SiW 12 O 40] 4- , [BW 12 O 40 ] 5- ) 经历第一个单电子还原波。然而,一旦 POM 进行双电子还原,就会与 NO 一起获得N 2 O。POM 第一个还原波的电位与其电荷相关。因此,对于 [PW 12 O 40 ] 3-和 [SiW 12 O 40 ] 4-和 [BW 12 O 40 ] 5-和 [H 2 W 12 O 40 ],NO 和/或 N 2 O 出现在较低的过电位下6-。实验的电流-电位曲线Microkinetic模拟被用于估计速率常数ķ Ç减小的POM和HNO之间的反应的2或NO 2 -在溶液和转换频率(TOF)。在低过电位下,TOF 值随着 POM 的氧化还原电位呈下降趋势:[PW 12 O 40 ] 3- > [SiW 12 O 40 ] 4- > [BW 12 O 40 ] 5-而在高时TOF 由k c主导的过电位按照相反的顺序。pH 5 k c值比 pH 2 低约 70 倍。这归因于质子浓度的降低以及带负电荷的 POM n-和 NO 2 –之间的静电排斥。











































 京公网安备 11010802027423号
京公网安备 11010802027423号