Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-12-03 , DOI: 10.1016/j.csbj.2021.11.042 Pierpaolo Cacciotto 1 , Andrea Basciu 1 , Francesco Oliva 1 , Giuliano Malloci 1 , Martin Zacharias 2 , Paolo Ruggerone 1 , Attilio V Vargiu 1
|
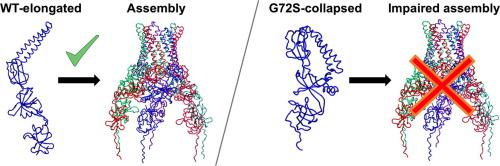
Efflux pumps of the Resistance-Nodulation-cell Division (RND) superfamily contribute to intrinsic and acquired resistance in Gram-negative pathogens by expelling chemically unrelated antibiotics with high efficiency. They are tripartite systems constituted by an inner-membrane-anchored transporter, an outer membrane factor protein, and a membrane fusion protein. Multimerization of the membrane fusion protein is an essential prerequisite for full functionality of these efflux pumps. In this work, we employed complementary computational techniques to investigate the stability of a dimeric unit of MexA (the membrane fusion protein of the MexAB-OprM RND efflux pump of Pseudomonas aeruginosa), and to provide a molecular rationale for the effect of the G72S substitution, which affects MexAB-OprM functionality by impairing the assembly of MexA. Our findings indicate that: i) dimers of this protein are stable in multiple µs-long molecular dynamics simulations; ii) the mutation drastically alters the conformational equilibrium of MexA, favouring a collapsed conformation that is unlikely to form dimers or higher order assemblies. Unveiling the mechanistic aspects underlying large conformational distortions induced by minor sequence changes is informative to efforts at interfering with the activity of this elusive bacterial weapon. In this respect, our work further confirms how molecular simulations can give important contribution and useful insights to characterize the mechanism of highly complex biological systems.
中文翻译:

MexA 中单个突变损害 MexAB-OprM 外排泵的分子原理
耐药结瘤细胞分裂 (RND) 超家族的外排泵通过高效排出化学上不相关的抗生素,导致革兰氏阴性病原体的内在和获得性耐药性。它们是由内膜锚定转运蛋白、外膜因子蛋白和膜融合蛋白构成的三方系统。膜融合蛋白的多聚化是这些外排泵完全发挥功能的必要先决条件。在这项工作中,我们采用互补的计算技术来研究 MexA 二聚体单元(铜绿假单胞菌的 MexAB-OprM RND 外排泵的膜融合蛋白)的稳定性),并为 G72S 取代的影响提供分子原理,该取代通过损害 MexA 的组装来影响 MexAB-OprM 功能。我们的研究结果表明:i)这种蛋白质的二聚体在多个微秒长的分子动力学模拟中是稳定的;ii) 突变极大地改变了 MexA 的构象平衡,有利于不太可能形成二聚体或更高阶装配体的折叠构象。揭示由微小序列变化引起的大构象扭曲背后的机制方面,有助于干扰这种难以捉摸的细菌武器的活动。在这方面,我们的工作进一步证实了分子模拟如何为表征高度复杂的生物系统的机制提供重要的贡献和有用的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号