当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Arylcarbamoylation of Alkenes of N-(o-Iodoaryl)acrylamides with Nitroarenes via Reductive Aminocarbonylation: Facile Synthesis of Carbamoyl-Substituted Oxindoles
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-03 , DOI: 10.1021/acs.orglett.1c03762 Chaozhihui Cheng 1, 2 , Jian-Nan Xiang 1 , Yan-Ping Zhu 3 , Zhi-Hong Peng 1 , Jin-Heng Li 1, 2, 3, 4
Organic Letters ( IF 4.9 ) Pub Date : 2021-12-03 , DOI: 10.1021/acs.orglett.1c03762 Chaozhihui Cheng 1, 2 , Jian-Nan Xiang 1 , Yan-Ping Zhu 3 , Zhi-Hong Peng 1 , Jin-Heng Li 1, 2, 3, 4
Affiliation
|
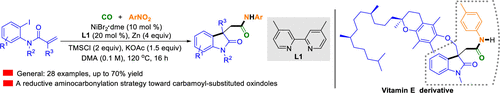
Nickel-catalyzed arylcarbamoylation reactions of alkenes of N-(o-haloaryl)acrylamides with CO and nitroarenes via reductive aminocarbonylation to produce carbamoyl-substituted oxindoles with an all-carbon quaternary stereogenic center are presented. Starting with N-(o-haloaryl)acrylamides, simple CO, and inexpensive nitroarenes and using a Ni catalyst, a dinitrogen-based ligand, a Zn reductant, a TMSCl additive, and a base system, this protocol enables the synthesis of various carbamoyl-substituted oxindoles and allows the efficient late-stage derivatization of valuable molecules.
中文翻译:

镍催化 N-(o-Iodoaryl) 丙烯酰胺烯烃与硝基芳烃通过还原氨基羰基化的芳基氨基甲酰化反应:氨基甲酰取代羟吲哚的简便合成
介绍了镍催化的N- ( o-卤代芳基) 丙烯酰胺烯烃与 CO 和硝基芳烃通过还原氨基羰基化反应生成具有全碳四元立体中心的氨基甲酰基取代的羟吲哚的芳基氨基甲酰化反应。从N-(邻卤代芳基)丙烯酰胺、简单的 CO 和廉价的硝基芳烃开始,并使用 Ni 催化剂、二氮基配体、Zn 还原剂、TMSCl 添加剂和基础系统,该协议能够合成各种氨基甲酰基-取代的羟吲哚并允许有价值分子的有效后期衍生化。
更新日期:2021-12-17
中文翻译:

镍催化 N-(o-Iodoaryl) 丙烯酰胺烯烃与硝基芳烃通过还原氨基羰基化的芳基氨基甲酰化反应:氨基甲酰取代羟吲哚的简便合成
介绍了镍催化的N- ( o-卤代芳基) 丙烯酰胺烯烃与 CO 和硝基芳烃通过还原氨基羰基化反应生成具有全碳四元立体中心的氨基甲酰基取代的羟吲哚的芳基氨基甲酰化反应。从N-(邻卤代芳基)丙烯酰胺、简单的 CO 和廉价的硝基芳烃开始,并使用 Ni 催化剂、二氮基配体、Zn 还原剂、TMSCl 添加剂和基础系统,该协议能够合成各种氨基甲酰基-取代的羟吲哚并允许有价值分子的有效后期衍生化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号