当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oriented Crystallization of Hydroxyapatite in Self-Assembled Peptide Fibrils as a Bonelike Material
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2021-12-02 , DOI: 10.1021/acsbiomaterials.1c00713 Changyu Shao 1, 2 , Zhisen Zhang 3 , Wenjing Jin 4 , Zhan Zhang 5 , Biao Jin 2 , Shuqin Jiang 1 , Haihua Pan 1 , Ruikang Tang 2 , James J De Yoreo 6 , Xiang Yang Liu 7
ACS Biomaterials Science & Engineering ( IF 5.4 ) Pub Date : 2021-12-02 , DOI: 10.1021/acsbiomaterials.1c00713 Changyu Shao 1, 2 , Zhisen Zhang 3 , Wenjing Jin 4 , Zhan Zhang 5 , Biao Jin 2 , Shuqin Jiang 1 , Haihua Pan 1 , Ruikang Tang 2 , James J De Yoreo 6 , Xiang Yang Liu 7
Affiliation
|
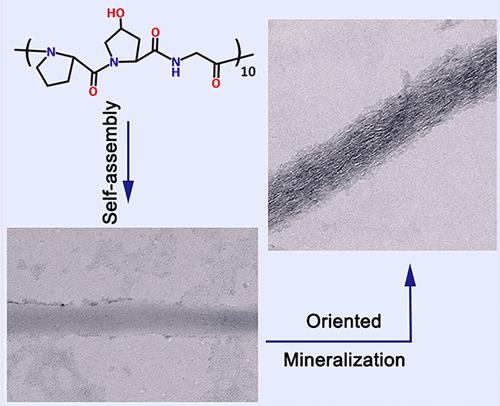
Controlling oriented crystallization is key to producing bonelike composite materials with a well-organized structure. However, producing this type of composite material using synthetic biopolymers as scaffolds is challenging. Inspired by the molecular structure of collagen-I, a collagenlike peptide─(Pro-Hyp-Gly)10 (POG10)─was designed to produce self-assembled fibrils that resemble the structure of collagen-I fibrils. In addition, the oriented mineralization of HAP crystals is formed in the fibrils that reproduces a bonelike material similar to collagen-I fibril mineralization. Unlike collagen-I fibrils, POG10 fibrils do not contain gap spaces. The molecular simulation results indicate that in addition to space confinement, the molecular field generated by POG10 can also confine the orientation of HAP, enriching our understanding of physical confinement and shedding light on the design of synthetic biopolymer scaffolds for bonelike material fabrication.
中文翻译:

自组装肽原纤维中羟基磷灰石的定向结晶作为类骨材料
控制定向结晶是生产具有良好组织结构的类骨复合材料的关键。然而,使用合成生物聚合物作为支架生产这种类型的复合材料具有挑战性。灵感来自胶原蛋白-I 的分子结构,一种胶原蛋白样肽─(Pro-Hyp-Gly) 10(POG10)─旨在生产类似于胶原蛋白-I 原纤维结构的自组装原纤维。此外,HAP 晶体的定向矿化在原纤维中形成,可再生类似于胶原蛋白-I 原纤维矿化的骨状材料。与胶原蛋白 I 原纤维不同,POG10 原纤维不包含间隙空间。分子模拟结果表明,除了空间限制外,POG10 产生的分子场还可以限制 HAP 的方向,丰富了我们对物理限制的理解,并为用于类骨材料制造的合成生物聚合物支架的设计提供了思路。
更新日期:2021-12-02
中文翻译:

自组装肽原纤维中羟基磷灰石的定向结晶作为类骨材料
控制定向结晶是生产具有良好组织结构的类骨复合材料的关键。然而,使用合成生物聚合物作为支架生产这种类型的复合材料具有挑战性。灵感来自胶原蛋白-I 的分子结构,一种胶原蛋白样肽─(Pro-Hyp-Gly) 10(POG10)─旨在生产类似于胶原蛋白-I 原纤维结构的自组装原纤维。此外,HAP 晶体的定向矿化在原纤维中形成,可再生类似于胶原蛋白-I 原纤维矿化的骨状材料。与胶原蛋白 I 原纤维不同,POG10 原纤维不包含间隙空间。分子模拟结果表明,除了空间限制外,POG10 产生的分子场还可以限制 HAP 的方向,丰富了我们对物理限制的理解,并为用于类骨材料制造的合成生物聚合物支架的设计提供了思路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号