当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 Solubility in Bis(trifluoromethylsulfonyl)imide ([Tf2N]) Anion-Based Ionic Liquids: [BVIM][Tf2N], [P4441][Tf2N], and [N4222][Tf2N]
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-01 , DOI: 10.1021/acs.jced.1c00433 Joon-Hyuk Yim 1 , Won-Wook Seo 1 , Jong Sung Lim 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-12-01 , DOI: 10.1021/acs.jced.1c00433 Joon-Hyuk Yim 1 , Won-Wook Seo 1 , Jong Sung Lim 1
Affiliation
|
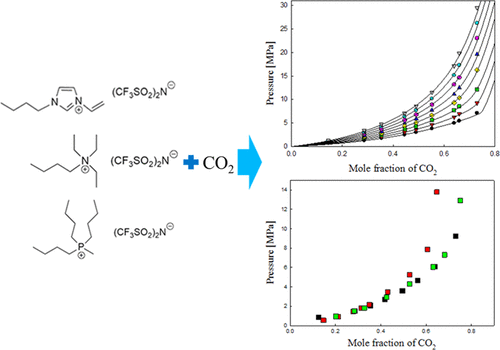
In this study, three types of [Tf2N]-based ionic liquids (ILs), 1-butyl-3-vinylimidazolium bis(trifluoromethylsulfonyl)imide ([BVIM][Tf2N]), butyltriethylammonium bis(trifluoromethylsulfonyl)imide ([N4222][Tf2N]), and tributylmethylphosphonium bis(trifluoromethylsulfonyl)imide ([P4441][Tf2N]), were selected to measure the solubility of CO2. A newly measured solubility database for these three ILs was generated in this study. It was found that the sequence of CO2 solubility was [BVIM][Tf2N] ≳ [P4441][Tf2N] > [N4222][Tf2N]. The CO2 solubility of the [BVIM] cation was approximately equal to that of the [P4441] cation at a low CO2 mole fraction region of less than 0.4; however, it was slightly higher than that of the [P4441] cation with increasing CO2 mole fraction. For the [N4222] cation, the CO2 solubility was similar to that of the [BVIM] and [P4441] cations when the CO2 mole fraction was less than 0.4; however, after this point, the CO2 solubility decreased drastically. To predict the ILs’ CO2 solubility at various temperature and composition ranges, thermodynamic modeling was conducted using the Peng–Robinson equation of state and the van der Waals one-fluid mixing rule. The critical properties and acentric factor of the ILs were calculated using the modified Lydersen–Joback–Reid method. The overall average absolute deviation of pressure (AAD-P) values were 0.0174, 0.0148, and 0.0153 for the CO2 + [BVIM][Tf2N], CO2 + [N4222][Tf2N], and CO2 + [P4441][Tf2N] systems, respectively. The values were estimated to be less than 2% on average for the three ILs, which indicated that the modeling equation fit well with the experimental data. In addition, Henry’s law constant was obtained using the extended Henry’s law at different temperatures. It should be noted that the constant increased with increasing temperature.
中文翻译:

双(三氟甲基磺酰基)亚胺 ([Tf2N]) 阴离子基离子液体中的 CO2 溶解度:[BVIM][Tf2N]、[P4441][Tf2N] 和 [N4222][Tf2N]
在这项研究中,三种类型的[Tf 2 N]基离子液体(ILs),1-丁基-3-乙烯基咪唑鎓双(三氟甲基磺酰基)亚胺([BVIM][Tf 2 N]),丁基三乙基铵双(三氟甲基磺酰基)亚胺(选择[N 4222 ][Tf 2 N])和三丁基甲基鏻双(三氟甲基磺酰基)亚胺([P 4441 ][Tf 2 N])来测量CO 2的溶解度。在本研究中生成了这三种 IL 的新测量的溶解度数据库。发现CO 2溶解度的顺序为[BVIM][Tf 2 N] ≳ [P 4441 ][Tf 2 N] > [N 4222 ][Tf2 N]。在低于 0.4的低 CO 2摩尔分数区域,[BVIM] 阳离子的 CO 2溶解度大约等于 [P 4441 ] 阳离子的溶解度;然而,随着CO 2摩尔分数的增加,它略高于[P 4441 ] 阳离子。对于[N 4222 ] 阳离子,当CO 2摩尔分数小于0.4时,CO 2溶解度与[BVIM]和[P 4441 ] 阳离子相似;然而,在此之后,CO 2溶解度急剧下降。预测 IL 的 CO 2在不同温度和组成范围内的溶解度,使用彭-罗宾逊状态方程和范德华单流体混合规则进行热力学建模。使用改进的 Lydersen-Joback-Reid 方法计算 ILs 的关键特性和无着丝粒因子。CO 2 + [BVIM][Tf 2 N]、CO 2 + [N 4222 ][Tf 2 N] 和 CO 2的总体平均压力绝对偏差 (AAD- P ) 值为 0.0174、0.0148 和0.0153 + [P 4441 ][Tf 2N]系统,分别。估计三个 IL 的值平均小于 2%,这表明建模方程与实验数据吻合良好。此外,亨利定律常数是在不同温度下使用扩展亨利定律获得的。需要注意的是,常数随着温度的升高而增加。
更新日期:2022-01-13
中文翻译:

双(三氟甲基磺酰基)亚胺 ([Tf2N]) 阴离子基离子液体中的 CO2 溶解度:[BVIM][Tf2N]、[P4441][Tf2N] 和 [N4222][Tf2N]
在这项研究中,三种类型的[Tf 2 N]基离子液体(ILs),1-丁基-3-乙烯基咪唑鎓双(三氟甲基磺酰基)亚胺([BVIM][Tf 2 N]),丁基三乙基铵双(三氟甲基磺酰基)亚胺(选择[N 4222 ][Tf 2 N])和三丁基甲基鏻双(三氟甲基磺酰基)亚胺([P 4441 ][Tf 2 N])来测量CO 2的溶解度。在本研究中生成了这三种 IL 的新测量的溶解度数据库。发现CO 2溶解度的顺序为[BVIM][Tf 2 N] ≳ [P 4441 ][Tf 2 N] > [N 4222 ][Tf2 N]。在低于 0.4的低 CO 2摩尔分数区域,[BVIM] 阳离子的 CO 2溶解度大约等于 [P 4441 ] 阳离子的溶解度;然而,随着CO 2摩尔分数的增加,它略高于[P 4441 ] 阳离子。对于[N 4222 ] 阳离子,当CO 2摩尔分数小于0.4时,CO 2溶解度与[BVIM]和[P 4441 ] 阳离子相似;然而,在此之后,CO 2溶解度急剧下降。预测 IL 的 CO 2在不同温度和组成范围内的溶解度,使用彭-罗宾逊状态方程和范德华单流体混合规则进行热力学建模。使用改进的 Lydersen-Joback-Reid 方法计算 ILs 的关键特性和无着丝粒因子。CO 2 + [BVIM][Tf 2 N]、CO 2 + [N 4222 ][Tf 2 N] 和 CO 2的总体平均压力绝对偏差 (AAD- P ) 值为 0.0174、0.0148 和0.0153 + [P 4441 ][Tf 2N]系统,分别。估计三个 IL 的值平均小于 2%,这表明建模方程与实验数据吻合良好。此外,亨利定律常数是在不同温度下使用扩展亨利定律获得的。需要注意的是,常数随着温度的升高而增加。











































 京公网安备 11010802027423号
京公网安备 11010802027423号