当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold(I)-Catalyzed Selective Cyclization and 1,2-Shift to Prepare Pseudorutaecarpine Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-11-30 , DOI: 10.1002/adsc.202101054 Wang WANG 1 , Nan-Ying CHEN 1 , Pei-Sen Zou 1 , Li PANG 1 , Dong-Liang Mo 1 , Cheng-Xue Pan 1 , Gui-Fa SU 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-11-30 , DOI: 10.1002/adsc.202101054 Wang WANG 1 , Nan-Ying CHEN 1 , Pei-Sen Zou 1 , Li PANG 1 , Dong-Liang Mo 1 , Cheng-Xue Pan 1 , Gui-Fa SU 1
Affiliation
|
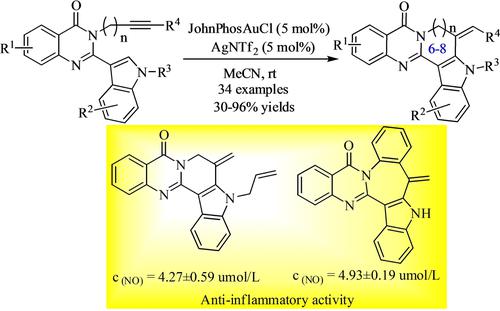
A variety of pseudorutaecarpine derivatives were prepared in good to excellent yields through a gold(I)-catalyzed selective cyclization and 1,2-shift of N-alkynyl quinazolinone-tethered indoles. Mechanistic study revealed that spiroindolenines generated in situ by cyclization at the the indole C3 position underwent an alkenyl 1,2-shift to generate pseudorutaecarpine. The reaction proceeds under mild reaction conditions, has a broad substrate scope, good functional group tolerance, and gram-scalable application. Furthermore, biological evaluation showed that most of the pseudorutaecarpine scaffolds prepared exhibit anti-inflammatory activity.
中文翻译:

金 (I) 催化选择性环化和 1,2-Shift 制备伪芸香碱衍生物
通过金 (I) 催化的选择性环化和N-炔基喹唑啉酮系链吲哚的 1,2-位移,以良好至优异的产率制备了多种假芸香碱衍生物。机理研究表明,通过在吲哚C3位环化原位产生的螺二氢吲哚经过烯基1,2-移位生成假芸香碱。该反应在温和的反应条件下进行,具有广泛的底物范围、良好的官能团耐受性和克级规模的应用。此外,生物学评估表明,大多数制备的假芸香碱支架具有抗炎活性。
更新日期:2021-11-30
中文翻译:

金 (I) 催化选择性环化和 1,2-Shift 制备伪芸香碱衍生物
通过金 (I) 催化的选择性环化和N-炔基喹唑啉酮系链吲哚的 1,2-位移,以良好至优异的产率制备了多种假芸香碱衍生物。机理研究表明,通过在吲哚C3位环化原位产生的螺二氢吲哚经过烯基1,2-移位生成假芸香碱。该反应在温和的反应条件下进行,具有广泛的底物范围、良好的官能团耐受性和克级规模的应用。此外,生物学评估表明,大多数制备的假芸香碱支架具有抗炎活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号