Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2021-11-30 , DOI: 10.1016/j.jcou.2021.101823 Marvin Oßkopp 1 , Armin Löwe 1 , Carlos M.S. Lobo 1 , Sebastian Baranyai 2 , Thulile Khoza 3 , Michael Auinger 4, 5 , Elias Klemm 1
|
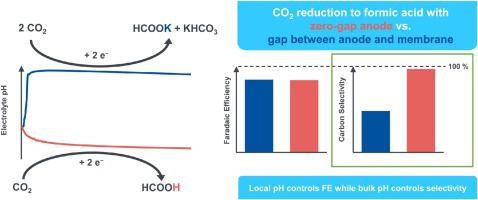
The electrochemical reduction of CO2 has the potential to become a key technology in the transformation to a sustainable circular carbon economy. Formic acid could be an important platform chemical for the eco-friendly chemical industries of tomorrow. However, in most cases the reduction of CO2 is performed in highly alkaline electrolyte systems yielding formates instead of formic acid. Furthermore, existing cell configurations do not allow an operation at acidic conditions below the pKa of formic acid over a long period of time. Both challenges make such a process unprofitable since they are associated with high costs for downstream processing. The main challenge, however, is the so-called “carbonate problem”, which is the non-faradaic formation of bicarbonate and carbonate from CO2 and OH− that cuts the carbon selectivity to faradaic products to half, even at 100 % faradaic efficiency. In the present work we evaluate three different cell configurations, using gas diffusion electrodes (GDEs) with a tin-oxide catalyst, at an industrially relevant current density of 200 mA cm-2. The quantification of outlet CO2 allows us to compare carbon selectivities, while the variation of current densities supported by numerical simulation results give insights into the local pH value inside the GDE. We demonstrate that an electrolyzer equipped with a single-layer GDE, a liquid electrolyte, and a zero-gap anode can achieve and sustain low pH values, especially below the pKa of formic acid. Altogether this paves the way for an industrial production of formic acid.
中文翻译:

通过电化学 CO2 还原在低 pH 值下生产甲酸
CO 2的电化学还原有可能成为向可持续循环碳经济转型的关键技术。甲酸可能成为未来环保化工行业的重要平台化学品。然而,在大多数情况下,CO 2的还原在高碱性电解质系统中进行,产生甲酸盐而不是甲酸。此外,现有的细胞配置不允许在低于 pKa的酸性条件下运行长时间使用甲酸。这两个挑战都使这种工艺无利可图,因为它们与下游加工的高成本有关。然而,主要挑战是所谓的“碳酸盐问题”,即从 CO 2和 OH -非法拉第形成碳酸氢盐和碳酸盐,即使在法拉第效率为 100 % 的情况下,也会将法拉第产物的碳选择性降低一半. 在目前的工作中,我们评估了三种不同的电池配置,使用气体扩散电极 (GDE) 和氧化锡催化剂,在工业相关的电流密度为 200 mA cm -2。出口CO 2的定量允许我们比较碳的选择性,而数值模拟结果支持的电流密度的变化可以让我们深入了解 GDE 内部的局部 pH 值。我们证明了配备单层 GDE、液体电解质和零间隙阳极的电解槽可以实现并维持低 pH 值,尤其是低于甲酸的 pKa。总之,这为甲酸的工业生产铺平了道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号