European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2021-11-30 , DOI: 10.1016/j.ejmech.2021.114021 Noha M Ibrahim 1 , Samar H Fahim 1 , Mariam Hassan 2 , Awatef E Farag 1 , Hanan H Georgey 3
|
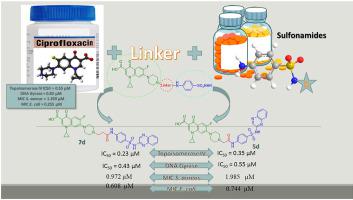
Fluoroquinolones are a class of antibacterial agents used clinically to treat a wide array of bacterial infections. Although being potent, susceptibility to CNS side effects limits their use. It was observed that improvements in absorption, activity and side effects were achieved via modifications at the N atom of the C7 of the side chain. To meet the increasing demand for development of new antibacterial agents, nineteen novel ciprofloxacin-sulfonamide hybrid molecules were designed, synthesized and characterized by IR, 1H NMR and 13C NMR as potential antibacterial agents with dual DNA gyrase/topoisomerase IV inhibitory activity. Most of the synthesized compounds showed significant antibacterial activity that was revealed by testing their inhibitory activity against DNA gyrase, DNA topoisomerase IV as well as their minimum inhibitory concentration against Staphylococcus aureus. Six ciprofloxacin-sulfonamide hybrids (3f, 5d, 7a, 7d, 7e and 9b) showed potent inhibitory activity against DNA topoisomerase IV, compared to ciprofloxacin (IC50: 0.55 μM), with IC50 range: 0.23–0.44 μM. DNA gyrase was also efficiently inhibited by five ciprofloxacin-sulfonamide hybrids (3f, 5d, 5e, 7a and 7d) with IC50 range: 0.43–1.1 μM (IC50 of ciprofloxacin: 0.83 μM). Compounds 3a and 3b showed a marked improvement in the antibacterial activity over ciprofloxacin against both Gram-positive and Gram-negative pathogens, namely, Staphylococcus aureus Newman and Escherichia coli ATCC8739, with MIC = 0.324 and 0.422 μM, respectively, that is 4.2-fold and 3.2-fold lower than ciprofloxacin (MIC = 1.359 μM) against the Gram-positive Staphylococcus aureus, and MIC = 0.025 and 0.013 μM, respectively, that is 10.2-fold and 19.6-fold lower than ciprofloxacin (MIC = 0.255 μM) against the Gram-negative Escherichia coli ATCC8739. Also, the most active compounds showed lower CNS and convulsive side effects compared to ciprofloxacin with a concomitant decrease in GABA expression.
中文翻译:

环丙沙星-磺胺杂化物的设计和合成以控制环丙沙星药理质量:效力和副作用
氟喹诺酮类药物是一类临床上用于治疗多种细菌感染的抗菌剂。尽管很有效,但对 CNS 副作用的敏感性限制了它们的使用。据观察,吸收、活性和副作用的改善是通过对侧链 C7 的 N 原子进行修饰来实现的。为满足新型抗菌药物开发日益增长的需求,设计、合成了 19 种新型环丙沙星-磺胺杂化分子,并通过 IR、1 H NMR 和13C NMR作为具有双重DNA旋转酶/拓扑异构酶IV抑制活性的潜在抗菌剂。大多数合成的化合物显示出显着的抗菌活性,通过测试它们对 DNA 促旋酶、DNA 拓扑异构酶 IV 的抑制活性以及它们对金黄色葡萄球菌的最小抑制浓度来揭示。与环丙沙星(IC50:0.55 μM)相比,六种环丙沙星-磺胺杂化物(3f、5d、7a、7d、7e和9b)对 DNA 拓扑异构酶 IV 显示出有效的抑制活性,IC50 范围为:0.23–0.44 μM。DNA 促旋酶也被五种环丙沙星-磺胺杂化物有效抑制。3f、5d、5e、7a和7d),IC50 范围:0.43–1.1 μM(环丙沙星的 IC50:0.83 μM)。化合物3a和3b对革兰氏阳性和革兰氏阴性病原体,即金黄色葡萄球菌 Newman和大肠杆菌 ATCC8739的抗菌活性比环丙沙星显着提高,MIC 分别为 0.324 和 0.422 μM,即 4.2 倍对革兰氏阳性金黄色葡萄球菌的作用比环丙沙星(MIC = 1.359 μM)低 3.2 倍,对革兰氏阳性葡萄球菌的 MIC = 0.025 和 0.013 μM,分别比环丙沙星(MIC = 0.255 μM)低 10.2 倍和 19.6 倍革兰氏阴性大肠杆菌 ATCC8739. 此外,与环丙沙星相比,最活跃的化合物显示出较低的中枢神经系统和惊厥副作用,同时 GABA 表达降低。











































 京公网安备 11010802027423号
京公网安备 11010802027423号