当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Time-dependent quantum dynamics study of the F + C2H6 → HF + C2H5 reaction
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-15 , DOI: 10.1039/d1cp04212b Delu Gao 1 , Dunyou Wang 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-15 , DOI: 10.1039/d1cp04212b Delu Gao 1 , Dunyou Wang 1
Affiliation
|
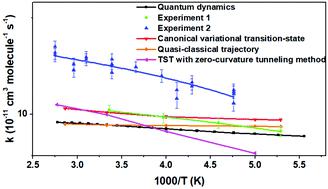
The reaction probabilities, integral cross sections, energy efficiency and rate constants are investigated for the F + C2H6 reaction using the quantum reaction dynamics, wave packet method. The ground-state integral cross section calculated using a six-degree-of-freedom approach is in very good agreement with the quasi-classical trajectory results. We find that the H–CH2CH3 stretching motion has the largest enhancement to reactivity, followed by the H–CH2–CH3 bending motion. However, the stretching motion between CH2 and CH3 slightly hinders the reactivity. The energy-form efficacy based on an equal amount of total energy shows that translational energy is more effective in enhancing the reactivity than vibrational energy of the H–CH2CH3 stretching motion at a relatively lower translational energy, while the reverse is true at a relatively high translational energy. An energy-shifting method is employed to calculate the full-dimensional rate constants. The quantum rate constants agree well with one of the two main experimental measurements, and the activation energy has an excellent agreement with the one calculated using canonical variational transition-state theory.
中文翻译:

F + C2H6 → HF + C2H5 反应的时间相关量子动力学研究
使用量子反应动力学、波包法研究了F + C 2 H 6反应的反应概率、积分截面、能量效率和速率常数。使用六自由度方法计算的基态积分截面与准经典轨迹结果非常吻合。我们发现 H–CH 2 CH 3拉伸运动对反应性的增强作用最大,其次是 H–CH 2 –CH 3弯曲运动。然而,CH 2和 CH 3之间的拉伸运动轻微阻碍反应性。基于等量总能量的能量形式效率表明,在相对较低的平移能量下,平移能比 H–CH 2 CH 3拉伸运动的振动能更有效地增强反应性,而在相对较高的平移能。采用能量转移方法计算全维速率常数。量子速率常数与两个主要实验测量值之一非常吻合,活化能与使用规范变分过渡态理论计算的活化能非常吻合。
更新日期:2021-11-26
中文翻译:

F + C2H6 → HF + C2H5 反应的时间相关量子动力学研究
使用量子反应动力学、波包法研究了F + C 2 H 6反应的反应概率、积分截面、能量效率和速率常数。使用六自由度方法计算的基态积分截面与准经典轨迹结果非常吻合。我们发现 H–CH 2 CH 3拉伸运动对反应性的增强作用最大,其次是 H–CH 2 –CH 3弯曲运动。然而,CH 2和 CH 3之间的拉伸运动轻微阻碍反应性。基于等量总能量的能量形式效率表明,在相对较低的平移能量下,平移能比 H–CH 2 CH 3拉伸运动的振动能更有效地增强反应性,而在相对较高的平移能。采用能量转移方法计算全维速率常数。量子速率常数与两个主要实验测量值之一非常吻合,活化能与使用规范变分过渡态理论计算的活化能非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号