当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From groove binding to intercalation: unravelling the weak interactions and other factors modulating the modes of interaction between methylated phenanthroline-based drugs and duplex DNA
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-02 , DOI: 10.1039/d1cp04529f Ángel Sánchez-González 1 , Tarsila G Castro 2 , Manuel Melle-Franco 3 , Adrià Gil 1, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-02 , DOI: 10.1039/d1cp04529f Ángel Sánchez-González 1 , Tarsila G Castro 2 , Manuel Melle-Franco 3 , Adrià Gil 1, 4
Affiliation
|
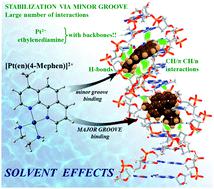
Several antitumor drugs base their cytotoxicity on their capacity to intercalate between base pairs of DNA. Nevertheless, it has been established that the mechanism of intercalation of drugs in DNA starts with the prior groove binding mode of interaction of the drug with DNA. Sometimes, for some kind of flat small molecules, groove binding does not produce any cytotoxic effect and the fast transition of such flat small molecules to the cytotoxic intercalation mode is desirable. This is the case of methylated phenanthroline (phen) derivatives, where, changes in the substitution in the position and number of methyl groups determine their capability as cytotoxic compounds and, therefore, it is a way for the modulation of cytotoxic effects. In this work, we studied this modulation by means of the interaction of the [Pt(en)(phen)]2+ complex and several derivatives by methylation of phen in different number and position and the d(GTCGAC)2 DNA hexamer via groove binding using PM6-DH2 and DFT-D methods. The analysis of the geometries, electronic structure and energetics of the studied systems was compared to experimental works to gain insight into the relation structure-interaction for the studied systems with cytotoxicity. The trends are explained by means of the Non-Covalent Interaction (NCI) index, the Energy Decomposition Analysis (EDA) and solvation contributions. Our results are in agreement with the experiments, in which the methylation of position 4 of phen seems to favour the interaction via groove binding thus making the transition to the intercalation cytotoxic mode difficult. Looking at the NCI results, these interactions come not only from the CH/π and CH/n interactions of the methyl group in position 4 but also from the ethylenediamine (en) ligand, whose orientation in the Pt complex was found in such a way that it produces a high number of weak interactions with DNA, especially with the sugar and phosphate backbone.
中文翻译:

从凹槽结合到嵌入:揭示弱相互作用和其他调节甲基化菲咯啉类药物与双链 DNA 之间相互作用模式的因素
几种抗肿瘤药物的细胞毒性基于它们插入 DNA 碱基对之间的能力。然而,已经确定药物在 DNA 中的嵌入机制始于药物与 DNA 相互作用的先前凹槽结合模式。有时,对于某种扁平小分子,凹槽结合不会产生任何细胞毒性作用,因此需要这种扁平小分子快速转变为细胞毒性嵌入模式。甲基化菲咯啉 (phen) 衍生物就是这种情况,其中甲基位置和数量的取代变化决定了它们作为细胞毒性化合物的能力,因此,它是一种调节细胞毒性作用的方法。在这项工作中,我们通过 [Pt(en)(phen)] 的相互作用研究了这种调制。使用 PM6-DH2 和 DFT-D 方法通过凹槽结合不同数量和位置的 phen 和 d(GTCGAC) 2 DNA 六聚体甲基化2+复合物和几种衍生物。将所研究系统的几何结构、电子结构和能量学分析与实验工作进行比较,以深入了解所研究系统与细胞毒性的关系结构-相互作用。通过非共价相互作用 (NCI) 指数、能量分解分析 (EDA) 和溶剂化贡献来解释趋势。我们的结果与实验一致,其中 phen 4 位的甲基化似乎有利于通过凹槽结合因此难以过渡到嵌入细胞毒性模式。查看 NCI 结果,这些相互作用不仅来自 4 位甲基的 CH/π 和 CH/n 相互作用,还来自乙二胺 (en) 配体,其在 Pt 配合物中的取向是以这种方式发现的它与 DNA 产生大量的弱相互作用,尤其是与糖和磷酸骨架的相互作用。
更新日期:2021-11-26
中文翻译:

从凹槽结合到嵌入:揭示弱相互作用和其他调节甲基化菲咯啉类药物与双链 DNA 之间相互作用模式的因素
几种抗肿瘤药物的细胞毒性基于它们插入 DNA 碱基对之间的能力。然而,已经确定药物在 DNA 中的嵌入机制始于药物与 DNA 相互作用的先前凹槽结合模式。有时,对于某种扁平小分子,凹槽结合不会产生任何细胞毒性作用,因此需要这种扁平小分子快速转变为细胞毒性嵌入模式。甲基化菲咯啉 (phen) 衍生物就是这种情况,其中甲基位置和数量的取代变化决定了它们作为细胞毒性化合物的能力,因此,它是一种调节细胞毒性作用的方法。在这项工作中,我们通过 [Pt(en)(phen)] 的相互作用研究了这种调制。使用 PM6-DH2 和 DFT-D 方法通过凹槽结合不同数量和位置的 phen 和 d(GTCGAC) 2 DNA 六聚体甲基化2+复合物和几种衍生物。将所研究系统的几何结构、电子结构和能量学分析与实验工作进行比较,以深入了解所研究系统与细胞毒性的关系结构-相互作用。通过非共价相互作用 (NCI) 指数、能量分解分析 (EDA) 和溶剂化贡献来解释趋势。我们的结果与实验一致,其中 phen 4 位的甲基化似乎有利于通过凹槽结合因此难以过渡到嵌入细胞毒性模式。查看 NCI 结果,这些相互作用不仅来自 4 位甲基的 CH/π 和 CH/n 相互作用,还来自乙二胺 (en) 配体,其在 Pt 配合物中的取向是以这种方式发现的它与 DNA 产生大量的弱相互作用,尤其是与糖和磷酸骨架的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号