当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ phosphonium-containing Lewis base-catalyzed 1,6-cyanation reaction: a facile way to obtain α-diaryl and α-triaryl acetonitriles
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-11 , DOI: 10.1039/d1qo01501j Jian-Ping Tan 1, 2 , Yuan Chen 2 , Xiaoyu Ren 2 , Yumeng Guo 1 , Bing Yi 1 , Hongkui Zhang 2 , Guowei Gao 2 , Tianli Wang 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-11 , DOI: 10.1039/d1qo01501j Jian-Ping Tan 1, 2 , Yuan Chen 2 , Xiaoyu Ren 2 , Yumeng Guo 1 , Bing Yi 1 , Hongkui Zhang 2 , Guowei Gao 2 , Tianli Wang 2
Affiliation
|
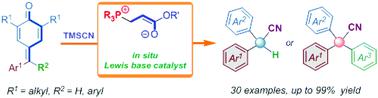
We present a phosphonium-containing catalyst generated in situ from phosphine and tert-butyl acrylate that serves as an unusual Lewis base catalyst. It was applied for the promotion of a remote 1,6-cyanation reaction of p-quinone methides and fuchsones employing trimethylsilyl cyanide as the cyanide source. A diverse range of α-diaryl and α-triaryl acetonitriles was obtained in high yields under mild reaction conditions with low catalyst loading (5 mol%). The practicality and utility of this protocol were demonstrated via the gram-scale preparation and facile elaboration of products. Mechanistic investigations (in situ NMR and ESI-MS analysis) were employed to characterize the active zwitterionic phosphonium intermediate, which was the “true” active catalyst.
中文翻译:

原位含鏻路易斯碱催化的1,6-氰化反应:一种获得α-二芳基和α-三芳基乙腈的简便方法
我们提出了一种由膦和丙烯酸叔丁酯原位生成的含鏻催化剂,作为一种不寻常的路易斯碱催化剂。它用于促进以氰化三甲基硅烷为氰化物源的对醌甲基化物和品红酮的远程 1,6-氰化反应。在温和的反应条件和低催化剂负载(5 mol%)下,以高产率获得了多种 α-二芳基和 α-三芳基乙腈。该协议的实用性和实用性通过克级制备和产品的简便加工得到了证明。机械调查(原位 NMR 和 ESI-MS 分析)用于表征活性两性离子鏻中间体,它是“真正的”活性催化剂。
更新日期:2021-12-01
中文翻译:

原位含鏻路易斯碱催化的1,6-氰化反应:一种获得α-二芳基和α-三芳基乙腈的简便方法
我们提出了一种由膦和丙烯酸叔丁酯原位生成的含鏻催化剂,作为一种不寻常的路易斯碱催化剂。它用于促进以氰化三甲基硅烷为氰化物源的对醌甲基化物和品红酮的远程 1,6-氰化反应。在温和的反应条件和低催化剂负载(5 mol%)下,以高产率获得了多种 α-二芳基和 α-三芳基乙腈。该协议的实用性和实用性通过克级制备和产品的简便加工得到了证明。机械调查(原位 NMR 和 ESI-MS 分析)用于表征活性两性离子鏻中间体,它是“真正的”活性催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号