当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Feasibility Study of Benzene Dehydration through an Adsorption Process: Isotherm Determination, Kinetics, and Fixed-Bed Column Studies
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-11-25 , DOI: 10.1021/acs.iecr.1c03081 Mehrnaz Joulazadeh 1 , Amir Rahimi 2 , S. Javad Mirmohammadi 1 , Masoud Kanani 1 , Saeed Dadkhah 1 , Mostafa Zarean 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2021-11-25 , DOI: 10.1021/acs.iecr.1c03081 Mehrnaz Joulazadeh 1 , Amir Rahimi 2 , S. Javad Mirmohammadi 1 , Masoud Kanani 1 , Saeed Dadkhah 1 , Mostafa Zarean 1
Affiliation
|
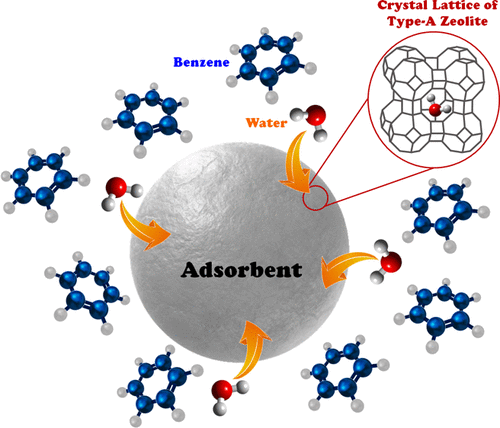
The benzene dehydration process is of vital significance in the industrial scale especially in linear alkyl benzene production plants. In the present work, different zeolites were utilized for elimination of water from benzene, and their water removal efficiencies were compared. The type of adsorption isotherm for the selected adsorbent (4A zeolite) was examined, and the experimental data were well-fitted with the Langmuir isotherm. The obtained results in kinetics studies suggested that the external and overall mass transfer coefficients are improved when the stirring rate was increased up to 100 rpm and remained constant with further increase in the agitation speed. Furthermore, a continuous setup was designed for investigating the effect of operational parameters on the breakthrough curve characteristics. The highest break time (612 min) was achieved when benzene’s superficial velocity and adsorption bed’s length were set at 0.002 m/s and 30 cm, respectively. The practicability of the 4A zeolite for commercial benzene dehydration applications was verified by its acceptable performance in the reusability study with slight efficiency reduction after four adsorption/desorption cycles. According to the obtained results from breakthrough curves, the internal and external mass transfer coefficients were estimated to be of the same order of magnitude, suggesting that both mass transfer mechanisms are important. Two well-known theoretical breakthrough models including Thomas and Adams–Bohart models were employed to describe the normalized concentration profiles and the predictions of the Thomas model fitted appropriately with the experimental results.
中文翻译:

通过吸附过程进行苯脱水的可行性研究:等温线测定、动力学和固定床柱研究
苯脱水工艺在工业规模尤其是直链烷基苯生产装置中具有重要意义。在目前的工作中,不同的沸石被用于去除苯中的水,并比较了它们的去水效率。检查了所选吸附剂(4A 沸石)的吸附等温线类型,实验数据与 Langmuir 等温线非常吻合。在动力学研究中获得的结果表明,当搅拌速率增加到 100 rpm 时,外部和整体传质系数得到改善,并随着搅拌速度的进一步增加而保持不变。此外,设计了一个连续设置来研究操作参数对突破曲线特性的影响。当苯的表观速度和吸附床长度分别设置为 0.002 m/s 和 30 cm 时,达到最长的断裂时间(612 分钟)。4A 沸石在商业苯脱水应用中的实用性通过其在可重复使用性研究中可接受的性能得到验证,在四次吸附/解吸循环后效率略有降低。根据突破曲线获得的结果,内部和外部传质系数估计为同一数量级,表明两种传质机制都很重要。采用包括 Thomas 和 Adams-Bohart 模型在内的两个著名的理论突破模型来描述归一化的浓度曲线,以及与实验结果适当拟合的 Thomas 模型的预测。
更新日期:2022-01-19
中文翻译:

通过吸附过程进行苯脱水的可行性研究:等温线测定、动力学和固定床柱研究
苯脱水工艺在工业规模尤其是直链烷基苯生产装置中具有重要意义。在目前的工作中,不同的沸石被用于去除苯中的水,并比较了它们的去水效率。检查了所选吸附剂(4A 沸石)的吸附等温线类型,实验数据与 Langmuir 等温线非常吻合。在动力学研究中获得的结果表明,当搅拌速率增加到 100 rpm 时,外部和整体传质系数得到改善,并随着搅拌速度的进一步增加而保持不变。此外,设计了一个连续设置来研究操作参数对突破曲线特性的影响。当苯的表观速度和吸附床长度分别设置为 0.002 m/s 和 30 cm 时,达到最长的断裂时间(612 分钟)。4A 沸石在商业苯脱水应用中的实用性通过其在可重复使用性研究中可接受的性能得到验证,在四次吸附/解吸循环后效率略有降低。根据突破曲线获得的结果,内部和外部传质系数估计为同一数量级,表明两种传质机制都很重要。采用包括 Thomas 和 Adams-Bohart 模型在内的两个著名的理论突破模型来描述归一化的浓度曲线,以及与实验结果适当拟合的 Thomas 模型的预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号