当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Diastereoselective Catalytic Approach to Pentasubstituted Pyrrolidines by Tandem Anionic-Radical Cross-Over Reactions
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-25 , DOI: 10.1002/adsc.202101172 Ullrich Jahn 1 , Denisa Hidasova 2 , Ivana Cisařová 3 , Radek Pohl 2
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2021-11-25 , DOI: 10.1002/adsc.202101172 Ullrich Jahn 1 , Denisa Hidasova 2 , Ivana Cisařová 3 , Radek Pohl 2
Affiliation
|
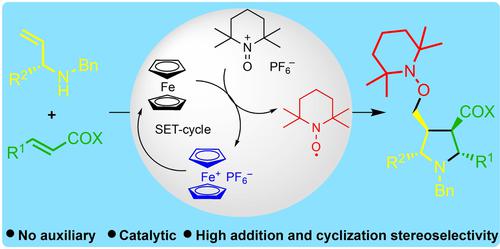
Pentasubstituted pyrrolidines are ubiquitous constituents of natural products and drugs, however the access options to them especially with respect to absolute and relative stereochemistry are not well developed. We report an asymmetric synthesis of N,2,3,4,5-pentasubstituted pyrrolidines by oxidative single-electron transfer-catalyzed tandem aza-Michael addition/radical 5-exo cyclization/oxygenation reactions. The absolute stereochemistry is set by applying readily accessible chiral allylamines in asymmetric conjugate additions to various β-substituted-α,β-unsaturated esters, which dependent on the substituent R2 sets the configuration of the two stereocenters next to the amine function, whereas two more are diastereoselectively generated by the radical 5-exo cyclization step. This allows the diastereodivergent single-step synthesis of pyrrolidines with four contiguous stereogenic centers. The stereoselectivity is rationalized by a predictive model.
中文翻译:

串联阴离子自由基交叉反应对五取代吡咯烷的非对映选择性催化方法
五取代的吡咯烷是天然产物和药物中普遍存在的成分,但是它们的获取选择,特别是在绝对和相对立体化学方面还没有得到很好的发展。我们报告了通过氧化单电子转移催化串联氮杂迈克尔加成/自由基5-外环化/氧化反应不对称合成N ,2,3,4,5-五取代吡咯烷。绝对立体化学是通过将易于获得的手性烯丙胺不对称共轭加成到各种 β-取代的-α,β-不饱和酯上来设定的,这取决于取代基 R 2设置胺功能旁边的两个立体中心的配置,而另外两个是由自由基 5-外环化步骤非对映选择性产生的。这允许非对映异构单步合成具有四个连续立体中心的吡咯烷。立体选择性通过预测模型合理化。
更新日期:2021-11-25
中文翻译:

串联阴离子自由基交叉反应对五取代吡咯烷的非对映选择性催化方法
五取代的吡咯烷是天然产物和药物中普遍存在的成分,但是它们的获取选择,特别是在绝对和相对立体化学方面还没有得到很好的发展。我们报告了通过氧化单电子转移催化串联氮杂迈克尔加成/自由基5-外环化/氧化反应不对称合成N ,2,3,4,5-五取代吡咯烷。绝对立体化学是通过将易于获得的手性烯丙胺不对称共轭加成到各种 β-取代的-α,β-不饱和酯上来设定的,这取决于取代基 R 2设置胺功能旁边的两个立体中心的配置,而另外两个是由自由基 5-外环化步骤非对映选择性产生的。这允许非对映异构单步合成具有四个连续立体中心的吡咯烷。立体选择性通过预测模型合理化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号