Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2021-11-26 , DOI: 10.1016/j.jhazmat.2021.127902 Tooba Saeed 1 , Abdul Naeem 1 , Israf Ud Din 2 , Muhammad Farooq 1 , Ihtisham Wali Khan 1 , Muhammad Hamayun 3 , Tabassum Malik 1
|
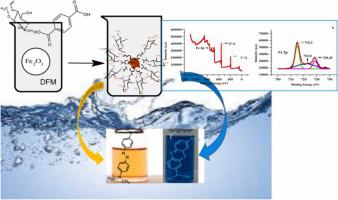
The iron metal-organic framework composite with chitosan (CS/MOF-235) was synthesized using a solvothermal method and its synthesis was confirmed by surface area, PZC, XRD, FESEM, XPS, TGA, TEM, EDX mapping and EDX analysis. The chitosan composite of the iron metal-organic framework (CS/MOF-235), MOF-235 and chitosan were used for the removal of methylene blue (MB) and methyl orange (MO) from aqueous solutions. The maximum adsorption capacities were found to be 2857 - 2326 mg/g for CS/MOF-235, 357 – 236 mg/g for MOF-235 and 209-171 mg/g for chitosan (CS) which reveal that the adsorption capacity of CS/MOF-235 is almost 8 and 14 times greater than MOF-235 and chitosan respectively. The adsorption selectivity of the (CS/MOF-235) towards the dye was in the order MO > MB. Moreover, hydrogen bonding, pi-pi bonding, pore-filling, electrostatic interactions and chemisorption were proposed as possible mechanisms for the removal of dyes onto CS/MOF-235.
The intraparticle diffusion and Richenberg models confirmed that the adsorption process was jointly controlled by the pore and film diffusion. The negative values of the isosteric heat of adsorption (Δ) fall with surface coverage indicating that a lesser amount of heat is required for the greater uptake of dyes.
中文翻译:

吸附染料的金属有机骨架壳聚糖复合材料的合成;动力学和热力学方法
采用溶剂热法合成了具有壳聚糖的铁金属-有机骨架复合材料(CS/MOF-235),并通过比表面积、PZC、XRD、FESEM、XPS、TGA、TEM、EDX映射和EDX分析证实了其合成。铁金属-有机骨架 (CS/MOF-235)、MOF-235 和壳聚糖的壳聚糖复合材料用于去除水溶液中的亚甲蓝 (MB) 和甲基橙 (MO)。 CS/MOF-235的最大吸附容量为 2857 - 2326 mg/ g,MOF-235 和 209-171 的最大吸附容量为 357 - 236 mg/g mg/g 壳聚糖 (CS) 表明 CS/MOF-235 的吸附能力分别是 MOF-235 和壳聚糖的 8 倍和 14 倍。(CS/MOF-235) 对染料的吸附选择性顺序为 MO > MB。此外,氢键、pi-pi 键、孔填充、静电相互作用和化学吸附被认为是染料在 CS/MOF-235 上去除的可能机制。
颗粒内扩散和 Richenberg 模型证实吸附过程由孔和膜扩散共同控制。等量吸附热的负值(Δ) 随表面覆盖率下降,这表明需要较少量的热量来吸收更多的染料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号