Heart Rhythm ( IF 5.5 ) Pub Date : 2021-11-26 , DOI: 10.1016/j.hrthm.2021.11.026 Hongbo Xiong 1 , Xuemei Bai 1 , Zhuang Quan 1 , Dong Yu 1 , Hongfu Zhang 1 , Chi Zhang 1 , Lina Liang 1 , Yufeng Yao 1 , Qin Yang 2 , Zhijie Wang 3 , Longfei Wang 4 , Yuan Huang 5 , Hui Li 1 , Xiang Ren 1 , Xin Tu 1 , Tie Ke 1 , Chengqi Xu 1 , Qing K Wang 1
|
Background
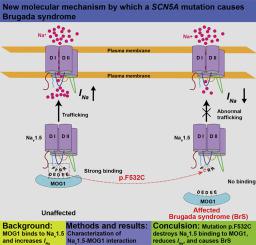
Mutations in cardiac sodium channel Nav1.5 cause Brugada syndrome (BrS). MOG1 is a chaperone that binds to Nav1.5, facilitates Nav1.5 trafficking to the cell surface, and enhances the amplitude of sodium current INa.
Objective
The purpose of this study was to identify structural elements involved in MOG1-Nav1.5 interaction and their relevance to the pathogenesis of BrS.
Methods
Systematic analyses of large deletions, microdeletions, and point mutations, and glutathione S-transferases pull-down, co-immunoprecipitation, cell surface protein quantification, and patch-clamping of INa were performed.
Results
Large deletion analysis defined the MOG1-Nav1.5 interaction domain to amino acids S476-H585 of Nav1.5 Loop I connecting transmembrane domains I and II. Microdeletion and point mutation analyses further defined the domain to F530T531F532R533R534R535. Mutations F530A, F532A, R533A, and R534A, but not T531A and R535A, significantly reduced MOG1-Nav1.5 interaction and eliminated MOG1-enhanced INa. Mutagenesis analysis identified D24, E36, D44, E53, and E101A of MOG1 as critical residues for interaction with Nav1.5 Loop I. We then characterized 3 mutations at the MOG1-Nav1.5 interaction domain: p.F530V, p.F532C, and p.R535Q reported from patients with long QT syndrome and BrS. We found that p.F532C reduced MOG1-Nav1.5 interaction and eliminated MOG1 function on INa; p.R535Q is also a loss-of-function mutation that reduces INa amplitude in a MOG1-independent manner, whereas p.F530V is benign as it does not have an apparent effect on MOG1 and INa.
Conclusion
Our findings define the MOG1-Nav1.5 interaction domain to a 5-amino-acid motif of F530T531F532R533R534 in Loop I. Mutation p.F532C associated with BrS abolishes Nav1.5 interaction with MOG1 and reduces MOG1-enhanced INa density, thereby uncovering a novel molecular mechanism for the pathogenesis of BrS.
中文翻译:

对心脏钠通道 Nav1.5 与 MOG1 相互作用的机制见解以及 Brugada 综合征的新分子机制
背景
心脏钠通道 Na v 1.5 的突变导致 Brugada 综合征 (BrS)。MOG1 是一种分子伴侣,可与 Na v 1.5 结合,促进 Na v 1.5 向细胞表面的运输,并增强钠电流 I Na的幅度。
客观的
本研究的目的是确定参与 MOG1-Na v 1.5 相互作用的结构元素及其与 BrS 发病机制的相关性。
方法
对大缺失、微缺失和点突变以及谷胱甘肽 S-转移酶下拉、免疫共沉淀、细胞表面蛋白定量和 I Na膜片钳进行了系统分析。
结果
大缺失分析定义了 MOG1-Na v 1.5 与连接跨膜结构域 I 和 II的 Na v 1.5 Loop I 的氨基酸 S 476 -H 585的相互作用结构域。微缺失和点突变分析进一步定义了 F 530 T 531 F 532 R 533 R 534 R 535的结构域。突变 F530A、F532A、R533A 和 R534A,但不是 T531A 和 R535A,显着降低 MOG1-Na v 1.5 相互作用并消除 MOG1 增强 I Na。诱变分析确定 MOG1 的 D24、E36、D44、E53 和 E101A 是与 Na v相互作用的关键残基1.5 Loop I。然后,我们对 MOG1-Na v 1.5 相互作用域的 3 个突变进行了表征:p.F530V、p.F532C 和 p.R535Q 从长 QT 综合征和 BrS 患者报告。我们发现 p.F532C 降低了 MOG1-Na v 1.5 的相互作用并消除了 I Na上的 MOG1 功能;p.R535Q 也是一种功能缺失突变,它以不依赖 MOG1 的方式降低 I Na幅度,而 p.F530V 是良性的,因为它对 MOG1 和 I Na没有明显影响。
结论
我们的研究结果将 MOG1-Na v 1.5 相互作用域定义为 Loop I 中 F 530 T 531 F 532 R 533 R 534的 5-氨基酸基序。与 BrS 相关的突变 p.F532C 消除了 Na v 1.5 与 MOG1 的相互作用并减少MOG1 增强 I Na密度,从而揭示了 BrS 发病机制的新分子机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号