Cell ( IF 45.5 ) Pub Date : 2021-11-24 , DOI: 10.1016/j.cell.2021.11.001 Alastair J H Brown 1 , Sophie J Bradley 2 , Fiona H Marshall 1 , Giles A Brown 1 , Kirstie A Bennett 1 , Jason Brown 1 , Julie E Cansfield 1 , David M Cross 3 , Chris de Graaf 1 , Brian D Hudson 1 , Louis Dwomoh 4 , João M Dias 1 , James C Errey 1 , Edward Hurrell 1 , Jan Liptrot 1 , Giulio Mattedi 1 , Colin Molloy 4 , Pradeep J Nathan 5 , Krzysztof Okrasa 1 , Greg Osborne 1 , Jayesh C Patel 1 , Mark Pickworth 1 , Nathan Robertson 1 , Shahram Shahabi 6 , Christoffer Bundgaard 7 , Keith Phillips 6 , Lisa M Broad 6 , Anushka V Goonawardena 8 , Stephen R Morairty 8 , Michael Browning 9 , Francesca Perini 10 , Gerard R Dawson 11 , John F W Deakin 12 , Robert T Smith 1 , Patrick M Sexton 13 , Julie Warneck 14 , Mary Vinson 1 , Tim Tasker 1 , Benjamin G Tehan 1 , Barry Teobald 1 , Arthur Christopoulos 15 , Christopher J Langmead 16 , Ali Jazayeri 1 , Robert M Cooke 1 , Prakash Rucktooa 1 , Miles S Congreve 1 , Malcolm Weir 1 , Andrew B Tobin 4
|
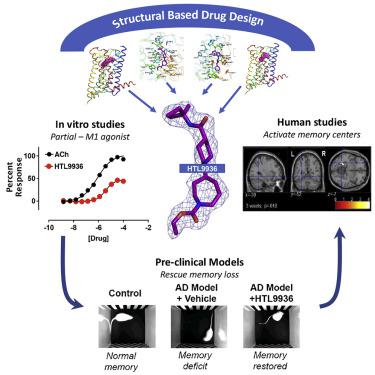
Current therapies for Alzheimer’s disease seek to correct for defective cholinergic transmission by preventing the breakdown of acetylcholine through inhibition of acetylcholinesterase, these however have limited clinical efficacy. An alternative approach is to directly activate cholinergic receptors responsible for learning and memory. The M1-muscarinic acetylcholine (M1) receptor is the target of choice but has been hampered by adverse effects. Here we aimed to design the drug properties needed for a well-tolerated M1-agonist with the potential to alleviate cognitive loss by taking a stepwise translational approach from atomic structure, cell/tissue-based assays, evaluation in preclinical species, clinical safety testing, and finally establishing activity in memory centers in humans. Through this approach, we rationally designed the optimal properties, including selectivity and partial agonism, into HTL9936—a potential candidate for the treatment of memory loss in Alzheimer’s disease. More broadly, this demonstrates a strategy for targeting difficult GPCR targets from structure to clinic.
中文翻译:

从结构到临床:具有治疗阿尔茨海默病潜力的毒蕈碱 M1 受体激动剂的设计
目前阿尔茨海默病的治疗方法试图通过抑制乙酰胆碱酯酶来防止乙酰胆碱的分解,从而纠正有缺陷的胆碱能传递,然而这些疗法的临床疗效有限。另一种方法是直接激活负责学习和记忆的胆碱能受体。 M1-毒蕈碱乙酰胆碱 (M1) 受体是首选靶点,但受到不良反应的阻碍。在这里,我们的目标是设计一种耐受性良好的 M1 激动剂所需的药物特性,通过从原子结构、基于细胞/组织的分析、临床前物种评估、临床安全性测试、最后在人类记忆中心建立活动。通过这种方法,我们合理地设计了HTL9936 的最佳特性,包括选择性和部分激动作用,HTL9936 是治疗阿尔茨海默病记忆丧失的潜在候选药物。更广泛地说,这展示了一种针对从结构到临床的困难 GPCR 靶标的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号