当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Increasing Current Density of Li-Mediated Ammonia Synthesis with High Surface Area Copper Electrodes
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-11-24 , DOI: 10.1021/acsenergylett.1c02104 Katja Li 1 , Sarah G. Shapel 1 , Degenhart Hochfilzer 1 , Jakob B. Pedersen 1 , Kevin Krempl 1 , Suzanne Z. Andersen 1 , Rokas Sažinas 1 , Mattia Saccoccio 1 , Shaofeng Li 1 , Debasish Chakraborty 1 , Jakob Kibsgaard 1 , Peter C. K. Vesborg 1 , Jens K. Nørskov 1 , Ib Chorkendorff 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2021-11-24 , DOI: 10.1021/acsenergylett.1c02104 Katja Li 1 , Sarah G. Shapel 1 , Degenhart Hochfilzer 1 , Jakob B. Pedersen 1 , Kevin Krempl 1 , Suzanne Z. Andersen 1 , Rokas Sažinas 1 , Mattia Saccoccio 1 , Shaofeng Li 1 , Debasish Chakraborty 1 , Jakob Kibsgaard 1 , Peter C. K. Vesborg 1 , Jens K. Nørskov 1 , Ib Chorkendorff 1
Affiliation
|
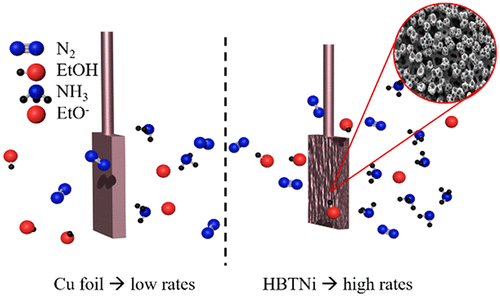
The lithium-mediated ammonia synthesis is so far the only proven electrochemical way to produce ammonia with promising faradaic efficiencies (FEs). However, to make this process commercially competitive, the ammonia formation rates per geometric surface area need to be increased significantly. In this study, we increased the current density by synthesizing high surface area Cu electrodes through hydrogen bubbling templating (HBT) on Ni foam substrates. With these electrodes, we achieved high ammonia formation rates of 46.0 ± 6.8 nmol s–1 cmgeo–2, at a current density of −100 mA/cmgeo–2 at 20 bar nitrogen atmosphere and comparable cell potentials to flat foil electrodes. The FE and energy efficiency (EE) under these conditions were 13.3 ± 2.0% and 2.3 ± 0.3%, respectively. Additionally, we found that increasing the electrolyte salt concentration improves the stability of the system, which is attributed to a change of Li deposition and/or solid electrolyte interphase.
中文翻译:

用高表面积铜电极提高锂介导氨合成的电流密度
迄今为止,锂介导的氨合成是唯一经过验证的电化学方法来生产具有有前景的法拉第效率 (FEs) 的氨。然而,为了使该工艺具有商业竞争力,需要显着提高每几何表面积的氨形成率。在这项研究中,我们通过在泡沫镍基板上进行氢气鼓泡模板 (HBT) 合成高表面积铜电极来增加电流密度。使用这些电极,我们实现了 46.0 ± 6.8 nmol s –1 cm geo –2的高氨生成速率,电流密度为 -100 mA/cm geo –2在 20 bar 的氮气气氛和与扁平箔电极相当的电池电位下。在这些条件下的 FE 和能效 (EE) 分别为 13.3 ± 2.0% 和 2.3 ± 0.3%。此外,我们发现增加电解质盐浓度提高了系统的稳定性,这归因于锂沉积和/或固体电解质界面的变化。
更新日期:2022-01-14
中文翻译:

用高表面积铜电极提高锂介导氨合成的电流密度
迄今为止,锂介导的氨合成是唯一经过验证的电化学方法来生产具有有前景的法拉第效率 (FEs) 的氨。然而,为了使该工艺具有商业竞争力,需要显着提高每几何表面积的氨形成率。在这项研究中,我们通过在泡沫镍基板上进行氢气鼓泡模板 (HBT) 合成高表面积铜电极来增加电流密度。使用这些电极,我们实现了 46.0 ± 6.8 nmol s –1 cm geo –2的高氨生成速率,电流密度为 -100 mA/cm geo –2在 20 bar 的氮气气氛和与扁平箔电极相当的电池电位下。在这些条件下的 FE 和能效 (EE) 分别为 13.3 ± 2.0% 和 2.3 ± 0.3%。此外,我们发现增加电解质盐浓度提高了系统的稳定性,这归因于锂沉积和/或固体电解质界面的变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号