当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potential energy profile for the Cl + (H2O)3 → HCl + (H2O)2OH reaction. A CCSD(T) study
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-12 , DOI: 10.1039/d1cp04309a Guoliang Li 1 , Ying Yao 1 , Shengyao Lü 1 , Yaoming Xie 2 , Gary E Douberly 2 , Henry F Schaefer 2
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-12 , DOI: 10.1039/d1cp04309a Guoliang Li 1 , Ying Yao 1 , Shengyao Lü 1 , Yaoming Xie 2 , Gary E Douberly 2 , Henry F Schaefer 2
Affiliation
|
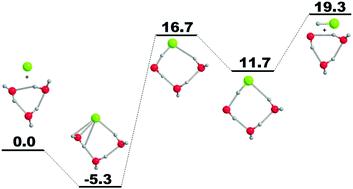
Four different reaction pathways are initially located for the reaction of Cl atom plus water trimer Cl + (H2O)3 → HCl + (H2O)2OH using a standard DFT method. As found for the analogous fluorine reaction, the geometrical and energetic results for the four chlorine pathways are closely related. However, the energetics for the Cl reaction are very different from those for fluorine. In the present paper, we investigate the lowest-energy chlorine pathway using the “gold standard” CCSD(T) method in conjunction with correlation-consistent basis sets up to cc-pVQZ. Structurally, the stationary points for the water trimer reaction Cl + (H2O)3 may be compared to those for the water monomer reaction Cl + H2O and water dimer reaction Cl + (H2O)2. Based on the CCSD(T) energies, the title reaction is endothermic by 19.3 kcal mol−1, with a classical barrier height of 16.7 kcal mol−1 between the reactants and the exit complex. There is no barrier for the reverse reaction. The Cl⋯(H2O)3 entrance complex lies 5.3 kcal mol−1 below the separated reactants. The HCl⋯(H2O)2OH exit complex is bound by 8.6 kcal mol−1 relative to the separated products. The Cl + (H2O)3 reaction is somewhat similar to the analogous Cl + (H2O)2 reaction, but qualitatively different from the Cl + H2O reaction. It is reasonable to expect that the reactions between the chlorine atom and larger water clusters may be similar to the Cl + (H2O)3 reaction. The potential energy profile for the Cl + (H2O)3 reaction is radically different from that for the valence isoelectronic F + (H2O)3 system, which may be related to the different bond energies between HCl and HF.
中文翻译:

Cl + (H2O)3 → HCl + (H2O)2OH 反应的势能分布。CCSD(T) 研究
使用标准 DFT 方法,最初为 Cl 原子加水三聚体 Cl + (H 2 O) 3 → HCl + (H 2 O) 2 OH的反应定位了四种不同的反应途径。对于类似的氟反应,四种氯途径的几何和能量结果密切相关。然而,Cl 反应的能量学与氟的能量学非常不同。在本论文中,我们使用“黄金标准”CCSD(T) 方法结合相关性一致的基础设置来研究最低能量氯途径,直至 cc-pVQZ。在结构上,水三聚体反应的静止点 Cl + (H 2 O) 3可以与水单体反应 Cl + H 2 O 和水二聚体反应 Cl + (H 2 O) 2 的那些进行比较。基于CCSD(T) 能量,标题反应吸热19.3 kcal mol -1,反应物和出口复合物之间的经典势垒高度为16.7 kcal mol -1。逆反应没有障碍。Cl⋯(H 2 O) 3入口复合物位于分离的反应物下方5.3 kcal mol -1处。相对于分离的产物,HCl⋯(H 2 O) 2 OH 出口复合物结合了 8.6 kcal mol -1。Cl + (H 2 O) 3反应有点类似于类似的 Cl + (H 2 O) 2反应,但与 Cl + H 2 O 反应在性质上不同。可以合理地预期氯原子和较大的水簇之间的反应可能类似于 Cl + (H 2 O) 3反应。Cl + (H 2 O) 3反应的势能曲线与价等电子F + (H 2 O) 3系统的势能曲线完全不同,这可能与HCl和HF之间不同的键能有关。
更新日期:2021-11-25
中文翻译:

Cl + (H2O)3 → HCl + (H2O)2OH 反应的势能分布。CCSD(T) 研究
使用标准 DFT 方法,最初为 Cl 原子加水三聚体 Cl + (H 2 O) 3 → HCl + (H 2 O) 2 OH的反应定位了四种不同的反应途径。对于类似的氟反应,四种氯途径的几何和能量结果密切相关。然而,Cl 反应的能量学与氟的能量学非常不同。在本论文中,我们使用“黄金标准”CCSD(T) 方法结合相关性一致的基础设置来研究最低能量氯途径,直至 cc-pVQZ。在结构上,水三聚体反应的静止点 Cl + (H 2 O) 3可以与水单体反应 Cl + H 2 O 和水二聚体反应 Cl + (H 2 O) 2 的那些进行比较。基于CCSD(T) 能量,标题反应吸热19.3 kcal mol -1,反应物和出口复合物之间的经典势垒高度为16.7 kcal mol -1。逆反应没有障碍。Cl⋯(H 2 O) 3入口复合物位于分离的反应物下方5.3 kcal mol -1处。相对于分离的产物,HCl⋯(H 2 O) 2 OH 出口复合物结合了 8.6 kcal mol -1。Cl + (H 2 O) 3反应有点类似于类似的 Cl + (H 2 O) 2反应,但与 Cl + H 2 O 反应在性质上不同。可以合理地预期氯原子和较大的水簇之间的反应可能类似于 Cl + (H 2 O) 3反应。Cl + (H 2 O) 3反应的势能曲线与价等电子F + (H 2 O) 3系统的势能曲线完全不同,这可能与HCl和HF之间不同的键能有关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号