当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper(II) ketimides in sp3 C–H amination
Chemical Science ( IF 7.6 ) Pub Date : 2021-11-05 , DOI: 10.1039/d1sc01990b Isuri U Jayasooriya 1 , Abolghasem Gus Bakhoda 1 , Rachel Palmer 1 , Kristi Ng 1 , Nour L Khachemoune 1 , Jeffery A Bertke 1 , Timothy H Warren 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-11-05 , DOI: 10.1039/d1sc01990b Isuri U Jayasooriya 1 , Abolghasem Gus Bakhoda 1 , Rachel Palmer 1 , Kristi Ng 1 , Nour L Khachemoune 1 , Jeffery A Bertke 1 , Timothy H Warren 1
Affiliation
|
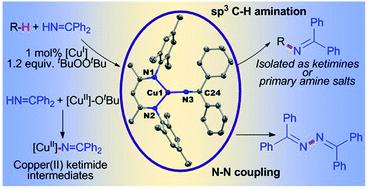
Commercially available benzophenone imine (HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2) reacts with β-diketiminato copper(II) tert-butoxide complexes [CuII]–OtBu to form isolable copper(II) ketimides [CuII]–N
CPh2) reacts with β-diketiminato copper(II) tert-butoxide complexes [CuII]–OtBu to form isolable copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2. Structural characterization of the three coordinate copper(II) ketimide [Me3NN]Cu–N
CPh2. Structural characterization of the three coordinate copper(II) ketimide [Me3NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 reveals a short Cu-Nketimide distance (1.700(2) Å) with a nearly linear Cu–N–C linkage (178.9(2)°). Copper(II) ketimides [CuII]–N
CPh2 reveals a short Cu-Nketimide distance (1.700(2) Å) with a nearly linear Cu–N–C linkage (178.9(2)°). Copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 readily capture alkyl radicals R˙ (PhCH(˙)Me and Cy˙) to form the corresponding R–N
CPh2 readily capture alkyl radicals R˙ (PhCH(˙)Me and Cy˙) to form the corresponding R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 products in a process that competes with N–N coupling of copper(II) ketimides [CuII]–N
CPh2 products in a process that competes with N–N coupling of copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 to form the azine Ph2C
CPh2 to form the azine Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2. Copper(II) ketimides [CuII]–N
CPh2. Copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 serve as intermediates in catalytic sp3 C–H amination of substrates R–H with ketimines HN
CAr2 serve as intermediates in catalytic sp3 C–H amination of substrates R–H with ketimines HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 and tBuOOtBu as oxidant to form N-alkyl ketimines R–N
CAr2 and tBuOOtBu as oxidant to form N-alkyl ketimines R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2. This protocol enables the use of unactivated sp3 C–H bonds to give R–N
CAr2. This protocol enables the use of unactivated sp3 C–H bonds to give R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 products easily converted to primary amines R–NH2 via simple acidic deprotection.
CAr2 products easily converted to primary amines R–NH2 via simple acidic deprotection.
中文翻译:

sp3 C-H 胺化中的铜 (II) 酮亚胺
市售的二苯甲酮亚胺 (HN![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2 ) 与 β-二酮亚胺铜 ( II )叔丁醇络合物 [Cu II ]–O t Bu 反应形成可分离的铜 ( II ) 酮亚胺 [Cu II ]–N
CPh 2 ) 与 β-二酮亚胺铜 ( II )叔丁醇络合物 [Cu II ]–O t Bu 反应形成可分离的铜 ( II ) 酮亚胺 [Cu II ]–N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2。三配位铜 ( II ) 酮亚胺 [Me 3 NN]Cu-N
CPh 2。三配位铜 ( II ) 酮亚胺 [Me 3 NN]Cu-N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2 的结构表征揭示了一个短的 Cu-N酮亚胺距离 (1.700(2) Å),具有接近线性的 Cu-N-C 键 (178.9(2) )°)。铜( II ) 酮亚胺 [Cu II ]–N
CPh 2 的结构表征揭示了一个短的 Cu-N酮亚胺距离 (1.700(2) Å),具有接近线性的 Cu-N-C 键 (178.9(2) )°)。铜( II ) 酮亚胺 [Cu II ]–N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2在与铜( II ) 酮亚胺 [Cu II ]-N CPh 2 的N-N 偶联竞争的过程中,容易捕获烷基自由基 R˙ (PhCH(˙)Me 和 Cy˙) 以形成相应的 R-N
CPh 2在与铜( II ) 酮亚胺 [Cu II ]-N CPh 2 的N-N 偶联竞争的过程中,容易捕获烷基自由基 R˙ (PhCH(˙)Me 和 Cy˙) 以形成相应的 R-N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2产物形成吖嗪 Ph 2 C N-N CPh 2。铜( II ) 酮亚胺 [Cu II ]-N CAr 2作为中间体催化 sp 3 C-H 胺化底物 R-H 与酮亚胺 HN CAr 2和t BuOO t Bu 作为氧化剂形成N-烷基酮亚胺 R-N
CPh 2产物形成吖嗪 Ph 2 C N-N CPh 2。铜( II ) 酮亚胺 [Cu II ]-N CAr 2作为中间体催化 sp 3 C-H 胺化底物 R-H 与酮亚胺 HN CAr 2和t BuOO t Bu 作为氧化剂形成N-烷基酮亚胺 R-N![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 车2。该协议允许使用未活化的 sp 3 C-H 键,通过简单的酸性脱保护,使 R-N
车2。该协议允许使用未活化的 sp 3 C-H 键,通过简单的酸性脱保护,使 R-N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr 2产品很容易转化为伯胺 R-NH 2 。
CAr 2产品很容易转化为伯胺 R-NH 2 。
更新日期:2021-11-24
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2) reacts with β-diketiminato copper(II) tert-butoxide complexes [CuII]–OtBu to form isolable copper(II) ketimides [CuII]–N
CPh2) reacts with β-diketiminato copper(II) tert-butoxide complexes [CuII]–OtBu to form isolable copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2. Structural characterization of the three coordinate copper(II) ketimide [Me3NN]Cu–N
CPh2. Structural characterization of the three coordinate copper(II) ketimide [Me3NN]Cu–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 reveals a short Cu-Nketimide distance (1.700(2) Å) with a nearly linear Cu–N–C linkage (178.9(2)°). Copper(II) ketimides [CuII]–N
CPh2 reveals a short Cu-Nketimide distance (1.700(2) Å) with a nearly linear Cu–N–C linkage (178.9(2)°). Copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 readily capture alkyl radicals R˙ (PhCH(˙)Me and Cy˙) to form the corresponding R–N
CPh2 readily capture alkyl radicals R˙ (PhCH(˙)Me and Cy˙) to form the corresponding R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 products in a process that competes with N–N coupling of copper(II) ketimides [CuII]–N
CPh2 products in a process that competes with N–N coupling of copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2 to form the azine Ph2C
CPh2 to form the azine Ph2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N
N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2. Copper(II) ketimides [CuII]–N
CPh2. Copper(II) ketimides [CuII]–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 serve as intermediates in catalytic sp3 C–H amination of substrates R–H with ketimines HN
CAr2 serve as intermediates in catalytic sp3 C–H amination of substrates R–H with ketimines HN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 and tBuOOtBu as oxidant to form N-alkyl ketimines R–N
CAr2 and tBuOOtBu as oxidant to form N-alkyl ketimines R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2. This protocol enables the use of unactivated sp3 C–H bonds to give R–N
CAr2. This protocol enables the use of unactivated sp3 C–H bonds to give R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr2 products easily converted to primary amines R–NH2 via simple acidic deprotection.
CAr2 products easily converted to primary amines R–NH2 via simple acidic deprotection.
中文翻译:

sp3 C-H 胺化中的铜 (II) 酮亚胺
市售的二苯甲酮亚胺 (HN
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2 ) 与 β-二酮亚胺铜 ( II )叔丁醇络合物 [Cu II ]–O t Bu 反应形成可分离的铜 ( II ) 酮亚胺 [Cu II ]–N
CPh 2 ) 与 β-二酮亚胺铜 ( II )叔丁醇络合物 [Cu II ]–O t Bu 反应形成可分离的铜 ( II ) 酮亚胺 [Cu II ]–N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2。三配位铜 ( II ) 酮亚胺 [Me 3 NN]Cu-N
CPh 2。三配位铜 ( II ) 酮亚胺 [Me 3 NN]Cu-N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2 的结构表征揭示了一个短的 Cu-N酮亚胺距离 (1.700(2) Å),具有接近线性的 Cu-N-C 键 (178.9(2) )°)。铜( II ) 酮亚胺 [Cu II ]–N
CPh 2 的结构表征揭示了一个短的 Cu-N酮亚胺距离 (1.700(2) Å),具有接近线性的 Cu-N-C 键 (178.9(2) )°)。铜( II ) 酮亚胺 [Cu II ]–N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2在与铜( II ) 酮亚胺 [Cu II ]-N CPh 2 的N-N 偶联竞争的过程中,容易捕获烷基自由基 R˙ (PhCH(˙)Me 和 Cy˙) 以形成相应的 R-N
CPh 2在与铜( II ) 酮亚胺 [Cu II ]-N CPh 2 的N-N 偶联竞争的过程中,容易捕获烷基自由基 R˙ (PhCH(˙)Me 和 Cy˙) 以形成相应的 R-N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh 2产物形成吖嗪 Ph 2 C N-N CPh 2。铜( II ) 酮亚胺 [Cu II ]-N CAr 2作为中间体催化 sp 3 C-H 胺化底物 R-H 与酮亚胺 HN CAr 2和t BuOO t Bu 作为氧化剂形成N-烷基酮亚胺 R-N
CPh 2产物形成吖嗪 Ph 2 C N-N CPh 2。铜( II ) 酮亚胺 [Cu II ]-N CAr 2作为中间体催化 sp 3 C-H 胺化底物 R-H 与酮亚胺 HN CAr 2和t BuOO t Bu 作为氧化剂形成N-烷基酮亚胺 R-N![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) 车2。该协议允许使用未活化的 sp 3 C-H 键,通过简单的酸性脱保护,使 R-N
车2。该协议允许使用未活化的 sp 3 C-H 键,通过简单的酸性脱保护,使 R-N ![[双键,长度为 m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CAr 2产品很容易转化为伯胺 R-NH 2 。
CAr 2产品很容易转化为伯胺 R-NH 2 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号