当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bispidine as a β-strand nucleator: from a β-arch to self-assembled cages and vesicles
Chemical Science ( IF 7.6 ) Pub Date : 2021-10-25 , DOI: 10.1039/d1sc04860k Hanuman Singh 1 , Akshay Chenna 2 , Upanshu Gangwar 1 , Julie Borah 2 , Gaurav Goel 2 , V Haridas 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-10-25 , DOI: 10.1039/d1sc04860k Hanuman Singh 1 , Akshay Chenna 2 , Upanshu Gangwar 1 , Julie Borah 2 , Gaurav Goel 2 , V Haridas 1
Affiliation
|
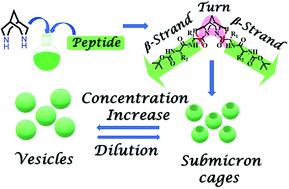
The development of synthetic scaffolds that nucleate well-folded secondary structures is highly challenging. Herein, we designed and synthesized a series of core-modified peptides (F1, F2, F3, and F4) that fold into β-strand structures. These bispidine-scaffolded peptides were studied by CD, IR, NMR, single crystal XRD, and Molecular Dynamics (MD) simulations to investigate their conformational preferences. Solid-state and solution studies revealed that bispidine is a versatile scaffold that could be placed either at the terminal or at the middle of the peptide strand for nucleating the β-strand structure. Scaffolds that nucleate an isolated β-strand conformation are rare. Bispidine placed at the C-terminus of the peptide chain could nucleate a β-strand conformation, while bispidine placed at the middle resulted in a β-arch conformation. This nucleation activity stems from the ability to restrict the psi torsion angle (ψ) through intramolecular C5 hydrogen bonding between the equatorial hydrogen(s) of bispidine and the carbonyl oxygen(s) of the amino acid close to the scaffold. Furthermore, the bispidine peptidomimetic with a super secondary structure, namely β-arch, assembled into single-hole submicron cages and spherical vesicles as evident from microscopic studies. The design logic defined here will be a significant strategy for the development of β-strand mimetics and super secondary structures.
中文翻译:

Bispidine 作为 β-链成核剂:从 β-拱形到自组装笼和囊泡
使折叠良好的二级结构成核的合成支架的开发极具挑战性。在此,我们设计并合成了一系列核心修饰肽(F1、F2、F3和F4) 折叠成 β 链结构。通过 CD、IR、NMR、单晶 XRD 和分子动力学 (MD) 模拟研究了这些双吡啶支架肽,以研究它们的构象偏好。固态和溶液研究表明,bispidine 是一种多功能支架,可以放置在肽链的末端或中间,以使 β 链结构成核。使分离的 β 链构象成核的支架很少见。位于肽链C-末端的Bispidine可以成核β-链构象,而位于中间的Bispidine会产生β-arch构象。这种成核活动源于限制 psi 扭转角 ( ψ) 通过双吡啶的赤道氢和靠近支架的氨基酸的羰基氧之间的分子内 C5 氢键。此外,具有超二级结构的双吡啶拟肽,即β-弓,组装成单孔亚微米笼和球形囊泡,从微观研究中可以看出。这里定义的设计逻辑将是开发 β 链模拟物和超二级结构的重要策略。
更新日期:2021-11-24
中文翻译:

Bispidine 作为 β-链成核剂:从 β-拱形到自组装笼和囊泡
使折叠良好的二级结构成核的合成支架的开发极具挑战性。在此,我们设计并合成了一系列核心修饰肽(F1、F2、F3和F4) 折叠成 β 链结构。通过 CD、IR、NMR、单晶 XRD 和分子动力学 (MD) 模拟研究了这些双吡啶支架肽,以研究它们的构象偏好。固态和溶液研究表明,bispidine 是一种多功能支架,可以放置在肽链的末端或中间,以使 β 链结构成核。使分离的 β 链构象成核的支架很少见。位于肽链C-末端的Bispidine可以成核β-链构象,而位于中间的Bispidine会产生β-arch构象。这种成核活动源于限制 psi 扭转角 ( ψ) 通过双吡啶的赤道氢和靠近支架的氨基酸的羰基氧之间的分子内 C5 氢键。此外,具有超二级结构的双吡啶拟肽,即β-弓,组装成单孔亚微米笼和球形囊泡,从微观研究中可以看出。这里定义的设计逻辑将是开发 β 链模拟物和超二级结构的重要策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号