当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Second-sphere effects on H2O2 activation by non-heme FeII complexes: role of a phenol group in the [H2O2]-dependent accumulation of FeIVO vs. FeIIIOOH
Chemical Science ( IF 7.6 ) Pub Date : 2021-11-17 , DOI: 10.1039/d1sc03303d Jean-Noël Rebilly 1 , Christian Herrero 1 , Katell Sénéchal-David 1 , Régis Guillot 1 , Tanya Inceoglu 1 , Hélène Maisonneuve 1 , Frédéric Banse 1
Chemical Science ( IF 7.6 ) Pub Date : 2021-11-17 , DOI: 10.1039/d1sc03303d Jean-Noël Rebilly 1 , Christian Herrero 1 , Katell Sénéchal-David 1 , Régis Guillot 1 , Tanya Inceoglu 1 , Hélène Maisonneuve 1 , Frédéric Banse 1
Affiliation
|
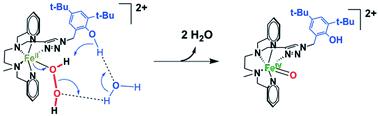
Redox metalloenzymes achieve very selective oxidation reactions under mild conditions using O2 or H2O2 as oxidants and release harmless side-products like water. Their oxidation selectivity is intrinsically linked to the control of the oxidizing species generated during the catalytic cycle. To do so, a second coordination sphere is used in order to create a pull effect during the activation of O2 or H2O2, thus ensuring a heterolytic O–O bond cleavage. Herein, we report the synthesis and study of a new non-heme FeII complex bearing a pentaazadentate first coordination sphere and a pendant phenol group. Its reaction with H2O2 generates the classical FeIIIOOH species at high H2O2 loading. But at low H2O2 concentrations, an FeIVO species is generated instead. The formation of the latter is directly related to the presence of the 2nd sphere phenol group. Kinetic, variable temperature and labelling studies support the involvement of the attached phenol as a second coordination sphere moiety (weak acid) during H2O2 activation. Our results suggest a direct FeII → FeIVO conversion directed by the 2nd sphere phenol via the protonation of the distal O atom of the FeII/H2O2 adduct leading to a heterolytic O–O bond cleavage.
中文翻译:

非血红素 FeII 复合物对 H2O2 活化的第二球效应:酚基在 FeIVO 与 FeIIIOOH 的 [H2O2] 依赖性积累中的作用
氧化还原金属酶使用O 2或H 2 O 2作为氧化剂在温和条件下实现非常选择性的氧化反应,并释放无害的副产物,例如水。它们的氧化选择性本质上与催化循环过程中产生的氧化物质的控制有关。为此,需要使用第二个配位球,以便在 O 2或 H 2 O 2活化过程中产生拉力效应,从而确保异裂 O-O 键断裂。在此,我们报道了一种新型非血红素 Fe II配合物的合成和研究,该配合物带有五氮齿第一配位球和侧苯酚基团。在高 H 2 O 2负载量下,它与 H 2 O 2反应生成经典的 Fe III OOH 物质。但在低 H 2 O 2浓度下,会生成 Fe IV O 物质。后者的形成与第二球酚基团的存在直接相关。动力学、可变温度和标记研究支持在 H 2 O 2活化过程中连接的苯酚作为第二配位球部分(弱酸)参与其中。 我们的结果表明,第二球苯酚通过Fe II /H 2 O 2加合物的远端 O 原子质子化直接进行 Fe II → Fe IV O 转化,导致异裂 O-O 键断裂。
更新日期:2021-11-24
中文翻译:

非血红素 FeII 复合物对 H2O2 活化的第二球效应:酚基在 FeIVO 与 FeIIIOOH 的 [H2O2] 依赖性积累中的作用
氧化还原金属酶使用O 2或H 2 O 2作为氧化剂在温和条件下实现非常选择性的氧化反应,并释放无害的副产物,例如水。它们的氧化选择性本质上与催化循环过程中产生的氧化物质的控制有关。为此,需要使用第二个配位球,以便在 O 2或 H 2 O 2活化过程中产生拉力效应,从而确保异裂 O-O 键断裂。在此,我们报道了一种新型非血红素 Fe II配合物的合成和研究,该配合物带有五氮齿第一配位球和侧苯酚基团。在高 H 2 O 2负载量下,它与 H 2 O 2反应生成经典的 Fe III OOH 物质。但在低 H 2 O 2浓度下,会生成 Fe IV O 物质。后者的形成与第二球酚基团的存在直接相关。动力学、可变温度和标记研究支持在 H 2 O 2活化过程中连接的苯酚作为第二配位球部分(弱酸)参与其中。 我们的结果表明,第二球苯酚通过Fe II /H 2 O 2加合物的远端 O 原子质子化直接进行 Fe II → Fe IV O 转化,导致异裂 O-O 键断裂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号