当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoproteomic Profiling by Cysteine Fluoroalkylation Reveals Myrocin G as an Inhibitor of the Nonhomologous End Joining DNA Repair Pathway
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-24 , DOI: 10.1021/jacs.1c09724 Daniel Abegg 1 , Martin Tomanik 2 , Nan Qiu 1 , Dany Pechalrieu 1 , Anton Shuster 1 , Bruno Commare 3 , Antonio Togni 3 , Seth B Herzon 2, 4 , Alexander Adibekian 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-24 , DOI: 10.1021/jacs.1c09724 Daniel Abegg 1 , Martin Tomanik 2 , Nan Qiu 1 , Dany Pechalrieu 1 , Anton Shuster 1 , Bruno Commare 3 , Antonio Togni 3 , Seth B Herzon 2, 4 , Alexander Adibekian 1
Affiliation
|
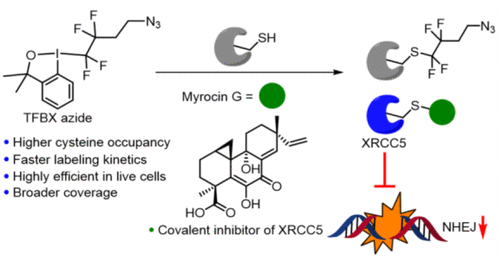
Chemoproteomic profiling of cysteines has emerged as a powerful method for screening the proteome-wide targets of cysteine-reactive fragments, drugs, and natural products. Herein, we report the development and an in-depth evaluation of a tetrafluoroalkyl benziodoxole (TFBX) as a cysteine-selective chemoproteomic probe. We show that this probe features numerous key improvements compared to the traditionally used cysteine-reactive probes, including a superior target occupancy, faster labeling kinetics, and broader proteomic coverage, thus enabling profiling of cysteines directly in live cells. In addition, the fluorine “signature” of probe 7 constitutes an additional advantage resulting in a more confident adduct–amino acid site assignment in mass-spectrometry-based identification workflows. We demonstrate the utility of our new probe for proteome-wide target profiling by identifying the cellular targets of (−)-myrocin G, an antiproliferative fungal natural product with a to-date unknown mechanism of action. We show that this natural product and a simplified analogue target the X-ray repair cross-complementing protein 5 (XRCC5), an ATP-dependent DNA helicase that primes DNA repair machinery for nonhomologous end joining (NHEJ) upon DNA double-strand breaks, making them the first reported inhibitors of this biomedically highly important protein. We further demonstrate that myrocins disrupt the interaction of XRCC5 with DNA leading to sensitization of cancer cells to the chemotherapeutic agent etoposide as well as UV-light-induced DNA damage. Altogether, our next-generation cysteine-reactive probe enables broader and deeper profiling of the cysteinome, rendering it a highly attractive tool for elucidation of targets of electrophilic small molecules.
中文翻译:

半胱氨酸氟烷基化化学蛋白质组学分析揭示 Myrocin G 作为非同源末端连接 DNA 修复途径的抑制剂
半胱氨酸的化学蛋白质组学分析已成为筛选半胱氨酸反应性片段、药物和天然产物的蛋白质组范围目标的有力方法。在此,我们报告了作为半胱氨酸选择性化学蛋白质组学探针的四氟烷基苯并氧唑 (TFBX) 的开发和深入评估。我们表明,与传统使用的半胱氨酸反应性探针相比,该探针具有许多关键改进,包括出色的目标占有率、更快的标记动力学和更广泛的蛋白质组学覆盖,从而能够直接在活细胞中分析半胱氨酸。此外,探针7的氟“签名”构成了一个额外的优势,导致在基于质谱的鉴定工作流程中更可靠的加合物 - 氨基酸位点分配。我们通过识别 (−)-myrocin G 的细胞靶标来证明我们的新探针在蛋白质组范围内的靶标分析中的实用性,这是一种具有迄今为止未知作用机制的抗增殖真菌天然产物。我们表明,这种天然产物和简化的类似物靶向 X 射线修复交叉互补蛋白5(XRCC5),一种 ATP 依赖性 DNA 解旋酶,在 DNA 双链断裂时启动 DNA 修复机制以进行非同源末端连接 (NHEJ),使其成为第一个报道的这种生物医学上非常重要的蛋白质的抑制剂。我们进一步证明 myrocins 破坏了 XRCC5 与 DNA 的相互作用,导致癌细胞对化疗剂依托泊苷以及紫外线诱导的 DNA 损伤敏感。总而言之,我们的下一代半胱氨酸反应性探针能够对半胱氨酸组进行更广泛和更深入的分析,使其成为阐明亲电子小分子目标的极具吸引力的工具。
更新日期:2021-12-08
中文翻译:

半胱氨酸氟烷基化化学蛋白质组学分析揭示 Myrocin G 作为非同源末端连接 DNA 修复途径的抑制剂
半胱氨酸的化学蛋白质组学分析已成为筛选半胱氨酸反应性片段、药物和天然产物的蛋白质组范围目标的有力方法。在此,我们报告了作为半胱氨酸选择性化学蛋白质组学探针的四氟烷基苯并氧唑 (TFBX) 的开发和深入评估。我们表明,与传统使用的半胱氨酸反应性探针相比,该探针具有许多关键改进,包括出色的目标占有率、更快的标记动力学和更广泛的蛋白质组学覆盖,从而能够直接在活细胞中分析半胱氨酸。此外,探针7的氟“签名”构成了一个额外的优势,导致在基于质谱的鉴定工作流程中更可靠的加合物 - 氨基酸位点分配。我们通过识别 (−)-myrocin G 的细胞靶标来证明我们的新探针在蛋白质组范围内的靶标分析中的实用性,这是一种具有迄今为止未知作用机制的抗增殖真菌天然产物。我们表明,这种天然产物和简化的类似物靶向 X 射线修复交叉互补蛋白5(XRCC5),一种 ATP 依赖性 DNA 解旋酶,在 DNA 双链断裂时启动 DNA 修复机制以进行非同源末端连接 (NHEJ),使其成为第一个报道的这种生物医学上非常重要的蛋白质的抑制剂。我们进一步证明 myrocins 破坏了 XRCC5 与 DNA 的相互作用,导致癌细胞对化疗剂依托泊苷以及紫外线诱导的 DNA 损伤敏感。总而言之,我们的下一代半胱氨酸反应性探针能够对半胱氨酸组进行更广泛和更深入的分析,使其成为阐明亲电子小分子目标的极具吸引力的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号