当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible or Irreversible Catalysis of H+/H2 Conversion by FeFe Hydrogenases
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-23 , DOI: 10.1021/jacs.1c09554 Andrea Fasano 1 , Henrik Land 2 , Vincent Fourmond 1 , Gustav Berggren 2 , Christophe Léger 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-23 , DOI: 10.1021/jacs.1c09554 Andrea Fasano 1 , Henrik Land 2 , Vincent Fourmond 1 , Gustav Berggren 2 , Christophe Léger 1
Affiliation
|
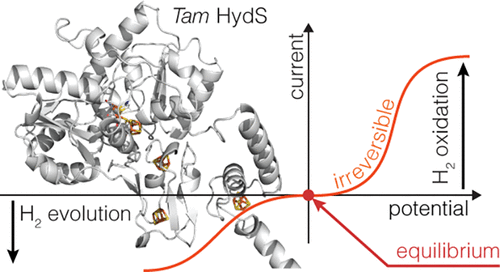
Studies of molecular catalysts traditionally aim at understanding how a certain mechanism allows the reaction to be fast. A distinct question, which has only recently received attention in the case of bidirectional molecular catalysts, is how much thermodynamic driving force is required to achieve fast catalysis in either direction of the reaction. “Reversible” catalysts are bidirectional catalysts that work either way in response to even a small departure from equilibrium and thus do not waste input free energy as heat; conversely, “irreversible” catalysts require a large driving force to proceed at an appreciable rate [Fourmond et al. Nat. Rev. Chem.2021, 5, 348–360]. Numerous mechanistic rationales for these contrasting behaviors have been proposed. To understand the determinants of catalytic (ir)reversibility, we examined the steady-state, direct electron transfer voltammetry of a particular FeFe hydrogenase, from Thermoanaerobacter mathranii, which is very unusual in that it irreversibly catalyzes H2 oxidation and production: a large overpotential is required for the reaction to proceed in either direction [Land et al. Chem. Sci.2020, 11, 12789–12801]. In contrast to previous hypotheses, we demonstrate that in this particular enzyme catalytic irreversibility can be explained without invoking slow interfacial electron transfer or variations in the mechanism: the observed kinetics is fully consistent with the same catalytic pathway being used in both directions of the reaction.
中文翻译:

FeFe氢化酶对H+/H2转化的可逆或不可逆催化
分子催化剂的研究传统上旨在了解某种机制如何使反应快速进行。一个独特的问题,最近才在双向分子催化剂的情况下受到关注,即需要多少热力学驱动力才能在反应的任一方向上实现快速催化。“可逆”催化剂是双向催化剂,可以响应即使是很小的平衡偏离,也可以双向工作,因此不会浪费输入的自由能作为热量;相反,“不可逆”催化剂需要很大的驱动力才能以可观的速度进行 [Fourmond 等人。纳特。牧师化学。2021 年,5, 348–360]。已经提出了针对这些对比行为的许多机械原理。为了了解催化 (ir) 可逆性的决定因素,我们检查了来自Thermoanaerobacter mathranii的特定 FeFe 氢化酶的稳态直接电子转移伏安法,这是非常不寻常的,因为它不可逆地催化 H 2氧化和产生:一个大的过电位反应在任一方向进行都需要 [Land et al. 化学。科学。2020 年11月, 12789–12801]。与以前的假设相反,我们证明了在这种特殊的酶中,催化不可逆性可以在不引起缓慢的界面电子转移或机制变化的情况下解释:观察到的动力学与在反应的两个方向上使用的相同催化途径完全一致。
更新日期:2021-12-08
中文翻译:

FeFe氢化酶对H+/H2转化的可逆或不可逆催化
分子催化剂的研究传统上旨在了解某种机制如何使反应快速进行。一个独特的问题,最近才在双向分子催化剂的情况下受到关注,即需要多少热力学驱动力才能在反应的任一方向上实现快速催化。“可逆”催化剂是双向催化剂,可以响应即使是很小的平衡偏离,也可以双向工作,因此不会浪费输入的自由能作为热量;相反,“不可逆”催化剂需要很大的驱动力才能以可观的速度进行 [Fourmond 等人。纳特。牧师化学。2021 年,5, 348–360]。已经提出了针对这些对比行为的许多机械原理。为了了解催化 (ir) 可逆性的决定因素,我们检查了来自Thermoanaerobacter mathranii的特定 FeFe 氢化酶的稳态直接电子转移伏安法,这是非常不寻常的,因为它不可逆地催化 H 2氧化和产生:一个大的过电位反应在任一方向进行都需要 [Land et al. 化学。科学。2020 年11月, 12789–12801]。与以前的假设相反,我们证明了在这种特殊的酶中,催化不可逆性可以在不引起缓慢的界面电子转移或机制变化的情况下解释:观察到的动力学与在反应的两个方向上使用的相同催化途径完全一致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号