当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Gold self-relay catalysis for accessing functionalized cyclopentenones bearing an all-carbon quaternary stereocenter
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-18 , DOI: 10.1039/d1qo01313k Fan-Tao Meng 1 , Jing-Long Chen 1 , Xiao-Yan Qin 1 , Tian-Shu Zhang 2 , Shu-Jiang Tu 1 , Bo Jiang 1 , Wen-Juan Hao 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-18 , DOI: 10.1039/d1qo01313k Fan-Tao Meng 1 , Jing-Long Chen 1 , Xiao-Yan Qin 1 , Tian-Shu Zhang 2 , Shu-Jiang Tu 1 , Bo Jiang 1 , Wen-Juan Hao 1
Affiliation
|
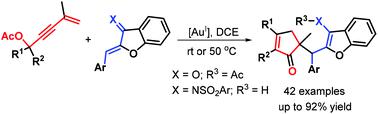
A new gold(I) self-relay catalysis consisting of a 3,3-rearrangement, Nazarov cyclization and Michael addition cascade of 1,3-enyne acetates with aurones and their derived azadienes is reported, and used to produce a series of densely functionalized cyclopentenones bearing a cyclic quaternary stereocenter in moderate to good yields under mild conditions. This tandem protocol demonstrates high regioselectivity, broad substrate flexibility and good functional group tolerance of substrates without inert atmosphere protection, providing a catalytic and convergent approach for creating all-carbon stereoscopic centers.
中文翻译:

用于获得带有全碳四元立体中心的功能化环戊烯酮的金自中继催化
报道了一种由 3,3-重排、Nazarov 环化和 1,3-烯炔乙酸酯与 aurones 及其衍生的氮杂二烯的迈克尔加成级联组成的新型金(I)自中继催化,并用于生产一系列密集功能化的环戊烯酮在温和条件下以中等至良好的产率具有环状四元立体中心。该串联方案展示了高区域选择性、广泛的底物灵活性和良好的底物官能团耐受性,无需惰性气氛保护,为创建全碳立体中心提供了催化和收敛的方法。
更新日期:2021-12-01
中文翻译:

用于获得带有全碳四元立体中心的功能化环戊烯酮的金自中继催化
报道了一种由 3,3-重排、Nazarov 环化和 1,3-烯炔乙酸酯与 aurones 及其衍生的氮杂二烯的迈克尔加成级联组成的新型金(I)自中继催化,并用于生产一系列密集功能化的环戊烯酮在温和条件下以中等至良好的产率具有环状四元立体中心。该串联方案展示了高区域选择性、广泛的底物灵活性和良好的底物官能团耐受性,无需惰性气氛保护,为创建全碳立体中心提供了催化和收敛的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号