Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlled delivery of gold nanoparticle-coupled miRNA therapeutics via an injectable self-healing hydrogel
Nanoscale ( IF 5.8 ) Pub Date : 2021-11-24 , DOI: 10.1039/d1nr04973a Casper F T van der Ven 1, 2, 3, 4 , Mark W Tibbitt 4, 5 , João Conde 6, 7 , Alain van Mil 1, 2, 8 , Jesper Hjortnaes 1, 2 , Pieter A Doevendans 2, 8 , Joost P G Sluijter 1, 2 , Elena Aikawa 3, 9 , Robert S Langer 4, 10
Nanoscale ( IF 5.8 ) Pub Date : 2021-11-24 , DOI: 10.1039/d1nr04973a Casper F T van der Ven 1, 2, 3, 4 , Mark W Tibbitt 4, 5 , João Conde 6, 7 , Alain van Mil 1, 2, 8 , Jesper Hjortnaes 1, 2 , Pieter A Doevendans 2, 8 , Joost P G Sluijter 1, 2 , Elena Aikawa 3, 9 , Robert S Langer 4, 10
Affiliation
|
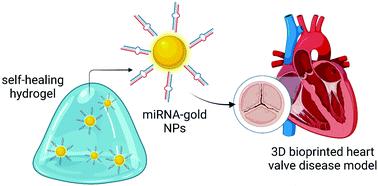
Differential expression of microRNAs (miRNAs) plays a role in many diseases, including cancer and cardiovascular diseases. Potentially, miRNAs could be targeted with miRNA-therapeutics. Sustained delivery of these therapeutics remains challenging. This study couples miR-mimics to PEG-peptide gold nanoparticles (AuNP) and loads these AuNP-miRNAs in an injectable, shear thinning, self-assembling polymer nanoparticle (PNP) hydrogel drug delivery platform to improve delivery. Spherical AuNPs coated with fluorescently labelled miR-214 are loaded into an HPMC-PEG-b-PLA PNP hydrogel. Release of AuNP/miRNAs is quantified, AuNP-miR-214 functionality is shown in vitro in HEK293 cells, and AuNP-miRNAs are tracked in a 3D bioprinted human model of calcific aortic valve disease (CAVD). Lastly, biodistribution of PNP-AuNP-miR-67 is assessed after subcutaneous injection in C57BL/6 mice. AuNP-miRNA release from the PNP hydrogel in vitro demonstrates a linear pattern over 5 days up to 20%. AuNP-miR-214 transfection in HEK293 results in 33% decrease of Luciferase reporter activity. In the CAVD model, AuNP-miR-214 are tracked into the cytoplasm of human aortic valve interstitial cells. Lastly, 11 days after subcutaneous injection, AuNP-miR-67 predominantly clears via the liver and kidneys, and fluorescence levels are again comparable to control animals. Thus, the PNP-AuNP-miRNA drug delivery platform provides linear release of functional miRNAs in vitro and has potential for in vivo applications.
中文翻译:

通过可注射的自愈水凝胶控制金纳米颗粒偶联 miRNA 疗法的传递
microRNA (miRNA) 的差异表达在许多疾病中发挥作用,包括癌症和心血管疾病。miRNA 疗法有可能成为靶点。持续提供这些疗法仍然具有挑战性。这项研究将 miR 模拟物与 PEG 肽金纳米颗粒 (AuNP) 结合,并将这些 AuNP-miRNA 加载到可注射、剪切稀化、自组装聚合物纳米颗粒 (PNP) 水凝胶药物递送平台中,以改善递送。将涂有荧光标记的 miR-214 的球形 AuNP 加载到 HPMC-PEG-b-PLA PNP 水凝胶中。对 AuNP/miRNA 的释放进行定量,在 HEK293 细胞中体外显示 AuNP-miR-214 的功能,并在钙化性主动脉瓣疾病 (CAVD) 的 3D 生物打印人类模型中跟踪 AuNP-miRNA。最后,在 C57BL/6 小鼠皮下注射后评估 PNP-AuNP-miR-67 的生物分布。体外PNP 水凝胶中 AuNP-miRNA 的释放在 5 天内呈线性模式,高达 20%。HEK293 中的 AuNP-miR-214 转染导致荧光素酶报告基因活性降低 33%。在 CAVD 模型中,AuNP-miR-214 被追踪到人主动脉瓣间质细胞的细胞质中。最后,皮下注射 11 天后,AuNP-miR-67 主要通过肝脏和肾脏清除,荧光水平再次与对照动物相当。因此,PNP-AuNP-miRNA 药物递送平台可在体外提供功能性 miRNA 的线性释放,并具有体内应用的潜力。
更新日期:2021-11-24
中文翻译:

通过可注射的自愈水凝胶控制金纳米颗粒偶联 miRNA 疗法的传递
microRNA (miRNA) 的差异表达在许多疾病中发挥作用,包括癌症和心血管疾病。miRNA 疗法有可能成为靶点。持续提供这些疗法仍然具有挑战性。这项研究将 miR 模拟物与 PEG 肽金纳米颗粒 (AuNP) 结合,并将这些 AuNP-miRNA 加载到可注射、剪切稀化、自组装聚合物纳米颗粒 (PNP) 水凝胶药物递送平台中,以改善递送。将涂有荧光标记的 miR-214 的球形 AuNP 加载到 HPMC-PEG-b-PLA PNP 水凝胶中。对 AuNP/miRNA 的释放进行定量,在 HEK293 细胞中体外显示 AuNP-miR-214 的功能,并在钙化性主动脉瓣疾病 (CAVD) 的 3D 生物打印人类模型中跟踪 AuNP-miRNA。最后,在 C57BL/6 小鼠皮下注射后评估 PNP-AuNP-miR-67 的生物分布。体外PNP 水凝胶中 AuNP-miRNA 的释放在 5 天内呈线性模式,高达 20%。HEK293 中的 AuNP-miR-214 转染导致荧光素酶报告基因活性降低 33%。在 CAVD 模型中,AuNP-miR-214 被追踪到人主动脉瓣间质细胞的细胞质中。最后,皮下注射 11 天后,AuNP-miR-67 主要通过肝脏和肾脏清除,荧光水平再次与对照动物相当。因此,PNP-AuNP-miRNA 药物递送平台可在体外提供功能性 miRNA 的线性释放,并具有体内应用的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号