Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rearrangement of protein structures on a gold nanoparticle surface is regulated by ligand adsorption modes
Nanoscale ( IF 5.8 ) Pub Date : 2021-10-27 , DOI: 10.1039/d1nr04813a Xiaofeng Wang 1 , Rong Lei 2 , Limei Li 1, 3 , Xinyu Fei 3 , Rui Ju 3 , Xiwen Sun 3 , Huiying Cao 3 , Qingfang Zhang 1 , Chunying Chen 4 , Xinyi Wang 1, 3
Nanoscale ( IF 5.8 ) Pub Date : 2021-10-27 , DOI: 10.1039/d1nr04813a Xiaofeng Wang 1 , Rong Lei 2 , Limei Li 1, 3 , Xinyu Fei 3 , Rui Ju 3 , Xiwen Sun 3 , Huiying Cao 3 , Qingfang Zhang 1 , Chunying Chen 4 , Xinyi Wang 1, 3
Affiliation
|
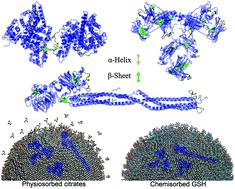
With development of the nanomedicine field and increasing hazards of exposure to nanobiological materials, research on the protein corona is urgently required. In particular, the understanding of the mechanism of structural changes of protein on a nanosurface should be improved. Herein, we focus on exploring the role of ligand adsorption modes (physiosorbed citrates or chemisorbed GSH) in the regulation of conformational rearrangement of three blood proteins (serum albumin, globulin, and fibrinogen) on the surface of gold nanoparticles. Through experimental measurements, protein adsorption features (thermodynamics, kinetics, adsorption orientation, and structural changes) were estimated. Molecular dynamics simulations further indicated that physiosorbed citrates could be gradually peeled off by approaching proteins and that the bare Au surface provided a stronger interface interaction than the chemisorbed GSH layer. Protein structure rearrangements were due to the reduction in protein internal energy, with an increase in H-bond formation involving a decrease in the α-helical content and an increase in the β-sheet content, to offset the high interfacial energy. Rearrangement of protein structures could occur either intramolecularly or intermolecularly. These findings enhanced our understanding of nano-protein interaction in the biological milieu and facilitate biomedical exploration of engineered nanomaterials.
中文翻译:

金纳米粒子表面蛋白质结构的重排受配体吸附模式的调节
随着纳米医学领域的发展和接触纳米生物材料的危害越来越大,迫切需要对蛋白质电晕进行研究。特别是,应提高对纳米表面蛋白质结构变化机制的理解。在此,我们重点探索配体吸附模式(物理吸附柠檬酸盐或化学吸附 GSH)在调节金纳米粒子表面三种血液蛋白质(血清白蛋白、球蛋白和纤维蛋白原)构象重排中的作用。通过实验测量,估计了蛋白质吸附特征(热力学、动力学、吸附方向和结构变化)。分子动力学模拟进一步表明,物理吸附的柠檬酸盐可以通过接近蛋白质逐渐剥离,并且裸露的 Au 表面提供了比化学吸附的 GSH 层更强的界面相互作用。蛋白质结构重排是由于蛋白质内能的降低,H键形成的增加,包括α-螺旋含量的减少和β-折叠含量的增加,以抵消高界面能。蛋白质结构的重排可以发生在分子内或分子间。这些发现增强了我们对生物环境中纳米蛋白质相互作用的理解,并促进了对工程纳米材料的生物医学探索。蛋白质结构重排是由于蛋白质内能的降低,H键形成的增加,包括α-螺旋含量的减少和β-折叠含量的增加,以抵消高界面能。蛋白质结构的重排可以发生在分子内或分子间。这些发现增强了我们对生物环境中纳米蛋白质相互作用的理解,并促进了对工程纳米材料的生物医学探索。蛋白质结构重排是由于蛋白质内能的降低,H键形成的增加,包括α-螺旋含量的减少和β-折叠含量的增加,以抵消高界面能。蛋白质结构的重排可以发生在分子内或分子间。这些发现增强了我们对生物环境中纳米蛋白质相互作用的理解,并促进了对工程纳米材料的生物医学探索。
更新日期:2021-11-24
中文翻译:

金纳米粒子表面蛋白质结构的重排受配体吸附模式的调节
随着纳米医学领域的发展和接触纳米生物材料的危害越来越大,迫切需要对蛋白质电晕进行研究。特别是,应提高对纳米表面蛋白质结构变化机制的理解。在此,我们重点探索配体吸附模式(物理吸附柠檬酸盐或化学吸附 GSH)在调节金纳米粒子表面三种血液蛋白质(血清白蛋白、球蛋白和纤维蛋白原)构象重排中的作用。通过实验测量,估计了蛋白质吸附特征(热力学、动力学、吸附方向和结构变化)。分子动力学模拟进一步表明,物理吸附的柠檬酸盐可以通过接近蛋白质逐渐剥离,并且裸露的 Au 表面提供了比化学吸附的 GSH 层更强的界面相互作用。蛋白质结构重排是由于蛋白质内能的降低,H键形成的增加,包括α-螺旋含量的减少和β-折叠含量的增加,以抵消高界面能。蛋白质结构的重排可以发生在分子内或分子间。这些发现增强了我们对生物环境中纳米蛋白质相互作用的理解,并促进了对工程纳米材料的生物医学探索。蛋白质结构重排是由于蛋白质内能的降低,H键形成的增加,包括α-螺旋含量的减少和β-折叠含量的增加,以抵消高界面能。蛋白质结构的重排可以发生在分子内或分子间。这些发现增强了我们对生物环境中纳米蛋白质相互作用的理解,并促进了对工程纳米材料的生物医学探索。蛋白质结构重排是由于蛋白质内能的降低,H键形成的增加,包括α-螺旋含量的减少和β-折叠含量的增加,以抵消高界面能。蛋白质结构的重排可以发生在分子内或分子间。这些发现增强了我们对生物环境中纳米蛋白质相互作用的理解,并促进了对工程纳米材料的生物医学探索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号