Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2021-12-31 , DOI: 10.2174/1573412917666210106115211 Fatang Yang 1 , Xiaoyun Duan 2 , Zhen Wang 1 , Yuming Dong 1
|
Aims: To establish a rapid and simultaneous determination of multiple effective ingredients in anti-cold drugs.
Background: Anti-cold drugs are stock medicines at home, and most anti-cold formulations are compound preparations. Although the active ingredients of compound preparations have significant effects on the treatment of colds, the excessive dosage or long-term use can produce a series of adverse reactions, including dependence, liver and kidney function damage, digestive system reaction, blood system damage. Now, there are many mature methods for analyzing the active ingredients of anti-cold drugs. However, these methods may have shortcomings, such as a long analysis time or a small number of analysis components.
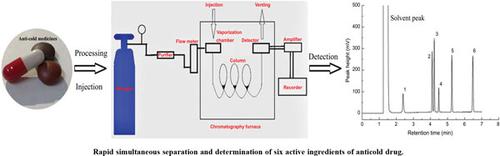
Objective: Establish a gas chromatography-flame ionization detector method for the simultaneous determination of six active ingredients, including acetaminophen, dextromethorphan hydrobromide, pseudoephedrine hydrochloride, chlorpheniramine maleate, diphenhydramine hydrochloride, and caffeine in anti-cold drugs.
Methods: After the standard was accurately weighed, dissolved in ethanol, filtered by 0.22 μm membrane and ultrasonically degassed, the gas chromatograph was used for detection. After the actual sample was removed from the coating, ground and crushed, accurately weighed, dissolved in ethanol, filtered by 0.22 μm membrane and ultrasonically degassed, the gas chromatograph was used for detection.
Results: The six components can be completely separated within 7.0min. This method has good sensitivity, precision, accuracy and recovery rate. Under the optimum testing conditions, the limit of detection was 0.360-2.50μg/mL, the limit of quantification was 1.20-8.30μg/mL. The calibration curves showed good linearity (R2≥0.9932) over the investigated concentration range between 1.20 and 400μg/mL. The recoveries were 89.2% to 109.2%. The RSD of intra-day precision was less than 1.0%. The RSD of inter-day precision was less than 3.2%. The established method was used to determine the ingredients of three anti-cold drugs on the market, and the results showed that the method can accurately determine the ingredients.
Conclusion: The method can quickly and simultaneously determine multiple active ingredients in anti-cold medicines. Compared with the published methods in literature, the proposed method has the advantages of fast, the number of analysis components wide application range, convenience, low cost, etc. It provides a reference method for quality control of active ingredients of anti-cold drugs.
中文翻译:

一种气相色谱火焰电离检测器快速同时分离测定抗感冒药六种活性成分的方法
目的:建立一种快速同时测定抗感冒药中多种有效成分的方法。
背景:抗感冒药是国内的库存药品,抗感冒药多为复方制剂。复方制剂的有效成分虽对治疗感冒有显着疗效,但过量或长期服用会产生一系列不良反应,包括依赖性、肝肾功能损害、消化系统反应、血液系统损害等。现在,分析抗感冒药有效成分的成熟方法有很多。然而,这些方法可能存在分析时间长或分析成分少等缺点。
目的:建立同时测定抗感冒药中对乙酰氨基酚、氢溴酸右美沙芬、盐酸伪麻黄碱、马来酸氯苯那敏、盐酸苯海拉明、咖啡因6种有效成分的气相色谱-火焰电离检测器方法。
方法:标准品准确称量,溶于乙醇,0.22 μm滤膜过滤,超声脱气后,气相色谱仪检测。将实际样品从涂层上取下,研磨粉碎,准确称量,溶于乙醇,0.22 μm滤膜过滤,超声脱气后,用气相色谱仪进行检测。
结果:6种组分可在7.0min内完全分离。该方法具有良好的灵敏度、精密度、准确度和回收率。在最佳检测条件下,检出限为0.360-2.50μg/mL,定量限为1.20-8.30μg/mL。校准曲线在 1.20 和 400μg/mL 之间的研究浓度范围内显示出良好的线性(R2≥0.9932)。回收率为 89.2% 至 109.2%。日内精密度的RSD小于1.0%。日间精密度的 RSD 小于 3.2%。将所建立的方法用于测定市售三种抗感冒药的成分,结果表明该方法能准确测定成分。
结论:该方法可快速、同时测定抗感冒药中的多种有效成分。与已发表的文献方法相比,该方法具有快速、分析组分数量多、适用范围广、方便、成本低等优点,为抗感冒药有效成分的质量控制提供了参考方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号