Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2021-12-31 , DOI: 10.2174/1573412917666201222100518 Lingling Xu 1 , Wenjun Zhou 1 , Yanjuan Yuan 2 , Qing Shao 2 , Hongqun Qiao 1
|
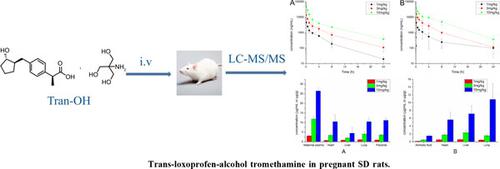
Background: As an anti-inflammatory prodrug, loxoprofen is metabolized into transloprofenol to treat diseases related to pain and inflammation. Although loxoprofen has fewer adverse effects than other NSAIDs, the safety of its usage during pregnancy remains unclear and needs to be considered. Fortunately, the toxicokinetics and tissue distribution study of transloxoprofen- alcohol in pregnant rats can resolve the problem.
Objective: The purpose of this study is to establish a simple, sensitive, and effective LC-MS/MS analysis method for determining the concentration of trans-loxoprofen alcohol in plasma and tissues.
Methods: The analytic samples were precipitated by methanol in one step and separated using a reverse-phase Poroshell 120 EC-C18 column (4.6 mm×50 mm; 2.7 μm). And the mobile gradient phase at a flow rate of 0.6 mL/min was composed of acetonitrile and 0.1% formic acid in water. The quantitative detection was achieved by multiple-reaction monitoring mode with a positive electrospray ionization source, transitional ion pairs of m/z 265.9>184.8 for trans-loxoprofenalcohol, and 268.8>187.9 for rac-trans-loxoprofen-D3 alcohol (Internal standard).
Results: A good linearity of calibration curves for plasma and tissues was observed in the concentration range from 5.0 to 5000 ng/mL, and the lower limit of quantification was detected at 5.0 ng/mL. The intra-day and inter-day precision in plasma and tissues were within 8.94% and 7.26%, respectively. The mean extraction recovery and matrix effects in plasma and tissues were in the range of 89.08~109.27% and 89.00~106.80%, respectively. Precision of stability in plasma and tissues was within 8.91% and 7.08%, respectively.
Conclusion: Complying with the requirements of bioanalytical guidelines by validation, this method was successfully adopted to the toxicokinetics and tissue distribution study after intravenously administrated trans-loxoprofen-alcohol into pregnant SD rats.
中文翻译:

使用经过验证的 LC-MS/MS 方法测定怀孕 SD 大鼠中的反式洛索洛芬酒精氨基丁三醇:在毒代动力学和组织分布研究中的应用
背景:作为一种抗炎前药,洛索洛芬被代谢为转洛洛芬醇以治疗与疼痛和炎症相关的疾病。尽管洛索洛芬的不良反应比其他NSAIDs少,但其在孕期使用的安全性尚不清楚,需要考虑。幸运的是,妊娠大鼠转洛索洛芬醇的毒代动力学和组织分布研究可以解决这个问题。
目的:本研究的目的是建立一种简单、灵敏、有效的LC-MS/MS分析方法,用于测定血浆和组织中反式洛索洛芬醇的浓度。
方法:分析样品用甲醇一步沉淀,并使用反相 Poroshell 120 EC-C18 柱(4.6 mm×50 mm;2.7 μm)进行分离。流速为 0.6 mL/min 的流动梯度相由乙腈和 0.1% 甲酸水溶液组成。定量检测采用多反应监测模式,正电喷雾电离源,过渡离子对m/z 265.9>184.8,反式洛索洛芬醇,268.8>187.9,外消旋-反式洛索洛芬-D3醇(内标) .
结果:血浆和组织的校准曲线在 5.0 至 5000 ng/mL 的浓度范围内具有良好的线性,在 5.0 ng/mL 处检测到定量下限。血浆和组织的日内和日间精密度分别在 8.94% 和 7.26% 以内。血浆和组织中的平均提取回收率和基质效应分别在89.08~109.27%和89.00~106.80%的范围内。血浆和组织中的稳定性精度分别在 8.91% 和 7.08% 以内。
结论:经验证符合生物分析指南的要求,该方法可成功应用于妊娠SD大鼠静脉注射反式洛索洛芬酒精后的毒代动力学和组织分布研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号