Current Pharmaceutical Analysis ( IF 0.7 ) Pub Date : 2021-12-31 , DOI: 10.2174/1573412917999201124143834 Houli Li 1 , Di Zhang 1 , Xiaoliang Cheng 1 , Qiaowei Zheng 1 , Kai Cheng 1 , Lilong Xiong 2 , Maoyi Wang 1 , Weihua Dong 1 , Weiyi Feng 1
|
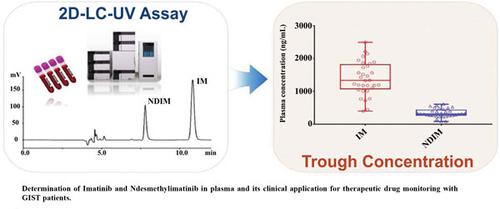
Background: The trough concentration (Cmin) of Imatinib (IM) is closely related to the treatment outcomes and adverse reactions of patients with gastrointestinal stromal tumors (GIST). However, the drug plasma level has great inter- and intra-individual variability, and therapeutic drug monitoring (TDM) is highly recommended.
Objective: To develop a novel, simple, and economical two-dimensional liquid chromatography method with the ultraviolet detector (2D-LC-UV) for simultaneous determination of IM and its major active metabolite, N-desmethyl imatinib (NDIM) in human plasma, and then apply the method for TDM of the drug.
Methods: The sample was processed by simple protein precipitation. Two target analytes were separated on the one-dimension column, captured on the middle column, and then transferred to the two-dimension column for further analysis. The detection was performed at 264 nm. The column temperature was maintained at 40˚C and the injection volume was 500 μL. Totally 32 plasma samples were obtained from patients with GIST who were receiving IM.
Results: IM and NDIM were separated well from other impurities and the entire analytical time for each run was 12.0 min. The calibration curves had good linearity in the range of 33.5-2678.4 ng/mL for IM, and 20.0-1600.0 ng/mL for NDIM, respectively. The extraction efficiency was more than 95%. The acceptable accuracy, precision, recovery and stability were also obtained. The Cmin of the drug in patients was measured with the validated method.
Conclusion: The novel 2D-LC-UV method is simple, stable, highly automated and independent of specialized technicians, which greatly increases the real-time capability of routine TDM for IM in hospital.
中文翻译:

一种同时测定血浆中伊马替尼和 N-去甲基伊马替尼的经验证的 2D-LC-UV 方法及其在 GIST 患者治疗药物监测中的临床应用
背景:伊马替尼(IM)的谷浓度(C min)与胃肠道间质瘤(GIST)患者的治疗结果和不良反应密切相关。然而,药物血浆水平具有很大的个体间和个体内变异性,强烈建议使用治疗药物监测 (TDM)。
目的:开发一种新型、简单、经济的二维液相色谱紫外检测器 (2D-LC-UV) 方法,用于同时测定人血浆中 IM 及其主要活性代谢物 N-去甲基伊马替尼 (NDIM),然后应用该方法进行药物的TDM。
方法:样品通过简单的蛋白质沉淀处理。两种目标分析物在一维柱上分离,在中间柱上捕获,然后转移到二维柱上进行进一步分析。检测在 264 nm 处进行。柱温保持在 40°C,进样量为 500 μL。从接受 IM 的 GIST 患者中获得了总共 32 份血浆样本。
结果: IM 和 NDIM 与其他杂质分离良好,每次运行的整个分析时间为 12.0 分钟。IM 的校准曲线分别在 33.5-2678.4 ng/mL 和 NDIM 的 20.0-1600.0 ng/mL 范围内具有良好的线性。提取效率达95%以上。还获得了可接受的准确度、精密度、回收率和稳定性。使用经过验证的方法测量患者中药物的 C min。
结论:新型二维-液相色谱-紫外方法简单、稳定、自动化程度高、独立于专业技术人员,大大提高了医院IM常规TDM的实时性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号