Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A buried glutamate in the cross-β core renders β-endorphin fibrils reversible
Nanoscale ( IF 5.8 ) Pub Date : 2021-11-08 , DOI: 10.1039/d1nr05679d Yuying Liu 1 , Yu Zhang 1 , Yunxiang Sun 1, 2 , Feng Ding 2
Nanoscale ( IF 5.8 ) Pub Date : 2021-11-08 , DOI: 10.1039/d1nr05679d Yuying Liu 1 , Yu Zhang 1 , Yunxiang Sun 1, 2 , Feng Ding 2
Affiliation
|
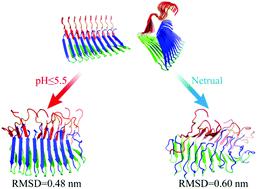
Functional amyloids are abundant in living organisms from prokaryotes to eukaryotes playing diverse biological roles. In contrast to the irreversible aggregation of most known pathological amyloids, we postulate that naturally-occurring functional amyloids are reversible under evolutionary pressure to be able to modulate the fibrillization process, reuse the composite peptides, or perform their biological functions. β-Endorphin, an endogenous opioid peptide hormone, forms such kinds of reversible amyloid fibrils in secretory granules for efficient storage and returns to the functional state of monomers upon release into the blood. The environmental change between low pH in secretory granules and neutral pH in extracellular spaces is believed to drive the reversible fibrillization of β-endorphin. Here, we investigate the critical role of a buried glutamate, Glu8, in the pH-responsive disassembly of β-endorphin fibrils using all-atom molecular dynamics simulations along with structure-based pKa prediction. The fibril was stable at pH 5.5 or lower with all the buried Glu8 residues protonated and neutrally charged. After switching to neutral pH, the Glu8 residues of peptides at the outer layers of the ordered fibrils became deprotonated due to partial solvent exposure, causing sheet-to-coil conformational changes and subsequent exposure of adjacent Glu8 residues in the inner chains. Via iterative deprotonation of Glu8 and induced structural disruption, all Glu8 residues would be progressively deprotonated. Electrostatic repulsion between deprotonated Glu8 residues along with their high solvation tendency disrupted the hydrogen bonding between the β1 strands and increased the solvent exposure of those otherwise buried residues in the cross-β core. Overall, our computational study reveals that the strategic positioning of ionizable residues into the cross-β core is a potential approach for designing reversible amyloid fibrils as pH-responsive smart bio-nanomaterials.
中文翻译:

交叉β核心中埋藏的谷氨酸使β-内啡肽原纤维可逆
功能性淀粉样蛋白在从原核生物到真核生物的活生物体中含量丰富,发挥着不同的生物学作用。与大多数已知的病理性淀粉样蛋白的不可逆聚集相反,我们假设天然存在的功能性淀粉样蛋白在进化压力下是可逆的,能够调节纤维化过程、重复使用复合肽或发挥其生物学功能。 β-内啡肽是一种内源性阿片肽激素,在分泌颗粒中形成此类可逆的淀粉样原纤维,以便有效储存,并在释放到血液中后恢复到单体的功能状态。分泌颗粒的低pH值和细胞外空间的中性pH值之间的环境变化被认为驱动β-内啡肽的可逆纤维化。在这里,我们利用全原子分子动力学模拟和基于结构的 p K a预测,研究了埋藏谷氨酸 Glu8 在 β-内啡肽原纤维 pH 响应性分解中的关键作用。原纤维在 pH 5.5 或更低时稳定,所有埋藏的 Glu8 残基均质子化并带中性电荷。切换到中性 pH 值后,有序原纤维外层的肽的 Glu8 残基由于部分溶剂暴露而去质子化,导致片状到卷曲的构象变化以及随后内链中相邻 Glu8 残基的暴露。通过Glu8 的迭代去质子化和诱导的结构破坏,所有 Glu8 残基将逐渐去质子化。 去质子化的 Glu8 残基之间的静电排斥及其高溶剂化倾向破坏了 β1 链之间的氢键,并增加了交叉 β 核心中那些原本埋藏的残基的溶剂暴露。总体而言,我们的计算研究表明,将可电离残基战略定位到交叉β核心中是设计可逆淀粉样原纤维作为pH响应型智能生物纳米材料的潜在方法。
更新日期:2021-11-23
中文翻译:

交叉β核心中埋藏的谷氨酸使β-内啡肽原纤维可逆
功能性淀粉样蛋白在从原核生物到真核生物的活生物体中含量丰富,发挥着不同的生物学作用。与大多数已知的病理性淀粉样蛋白的不可逆聚集相反,我们假设天然存在的功能性淀粉样蛋白在进化压力下是可逆的,能够调节纤维化过程、重复使用复合肽或发挥其生物学功能。 β-内啡肽是一种内源性阿片肽激素,在分泌颗粒中形成此类可逆的淀粉样原纤维,以便有效储存,并在释放到血液中后恢复到单体的功能状态。分泌颗粒的低pH值和细胞外空间的中性pH值之间的环境变化被认为驱动β-内啡肽的可逆纤维化。在这里,我们利用全原子分子动力学模拟和基于结构的 p K a预测,研究了埋藏谷氨酸 Glu8 在 β-内啡肽原纤维 pH 响应性分解中的关键作用。原纤维在 pH 5.5 或更低时稳定,所有埋藏的 Glu8 残基均质子化并带中性电荷。切换到中性 pH 值后,有序原纤维外层的肽的 Glu8 残基由于部分溶剂暴露而去质子化,导致片状到卷曲的构象变化以及随后内链中相邻 Glu8 残基的暴露。通过Glu8 的迭代去质子化和诱导的结构破坏,所有 Glu8 残基将逐渐去质子化。 去质子化的 Glu8 残基之间的静电排斥及其高溶剂化倾向破坏了 β1 链之间的氢键,并增加了交叉 β 核心中那些原本埋藏的残基的溶剂暴露。总体而言,我们的计算研究表明,将可电离残基战略定位到交叉β核心中是设计可逆淀粉样原纤维作为pH响应型智能生物纳米材料的潜在方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号