当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamic properties of a molecular dipolar liquid using the two-phase thermodynamic approach
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-03 , DOI: 10.1039/d1cp03246a Ricardo Palomar 1 , Gemma Sesé 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2021-11-03 , DOI: 10.1039/d1cp03246a Ricardo Palomar 1 , Gemma Sesé 1
Affiliation
|
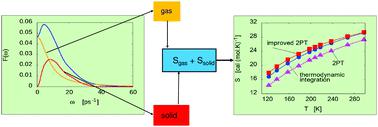
A modified version of the original two-phase thermodynamic approach has been extended to evaluate the thermodynamic properties of molecular systems. Its basic assumption states that the density of states can be decomposed into solid-like and gas-like components. The solid part has been approximated by that of a set of harmonic oscillators, whereas a subset composed of rough hard spheres has been considered for the gas part. In this new approach, molecules have been modelled as rotating hard spheres that experience elastic collisions. The technique has been tested on a system made up of dipolar diatomic molecules, and it leads to very good results for total entropy, potential energy reference and heat capacity. Translation and rotation solid components of the overall spectra have been compared to the real part of the instantaneous normal mode vibrational densities of states. Similarities between them reinforce the validity of the two-phase thermodynamic approach.
中文翻译:

使用两相热力学方法的分子偶极液体的热力学性质
原始两相热力学方法的修改版本已扩展到评估分子系统的热力学性质。它的基本假设指出,态密度可以分解为类固体和类气体的成分。固体部分已被一组谐振子近似,而气体部分已考虑由粗糙的硬球组成的子集。在这种新方法中,分子被建模为经历弹性碰撞的旋转硬球。该技术已经在由偶极双原子分子组成的系统上进行了测试,它在总熵、势能参考和热容方面取得了非常好的结果。已经将整个光谱的平移和旋转固体分量与状态的瞬时正常模式振动密度的实部进行了比较。它们之间的相似性加强了两相热力学方法的有效性。
更新日期:2021-11-22
中文翻译:

使用两相热力学方法的分子偶极液体的热力学性质
原始两相热力学方法的修改版本已扩展到评估分子系统的热力学性质。它的基本假设指出,态密度可以分解为类固体和类气体的成分。固体部分已被一组谐振子近似,而气体部分已考虑由粗糙的硬球组成的子集。在这种新方法中,分子被建模为经历弹性碰撞的旋转硬球。该技术已经在由偶极双原子分子组成的系统上进行了测试,它在总熵、势能参考和热容方面取得了非常好的结果。已经将整个光谱的平移和旋转固体分量与状态的瞬时正常模式振动密度的实部进行了比较。它们之间的相似性加强了两相热力学方法的有效性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号