Advanced Drug Delivery Reviews ( IF 15.2 ) Pub Date : 2021-11-20 , DOI: 10.1016/j.addr.2021.114066 Yuncheng Wang 1 , Rachel Yoon Kyung Chang 1 , Warwick J Britton 2 , Hak-Kim Chan 1
|
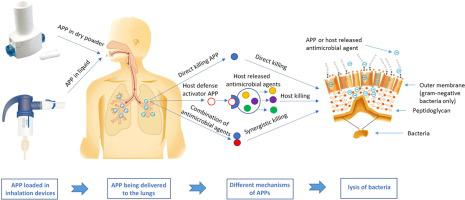
Antimicrobial peptides and proteins (APPs) are becoming increasingly important in targeting multidrug-resistant (MDR) bacteria. APPs is a rapidly emerging area with novel molecules being produced and further optimised to enhance antimicrobial efficacy, while overcoming issues associated with biologics such as potential toxicity and low bioavailability resulting from short half-life. Inhalation delivery of these agents can be an effective treatment of respiratory infections owing to the high local drug concentration in the lungs with lower exposure to systemic circulation hence reducing systemic toxicity. This review describes the recent studies on inhaled APPs, including in vitro and in vivo antimicrobial activities, toxicity assessments, and formulation strategies whenever available. The review also includes studies on combination of APPs with other antimicrobial agents to achieve enhanced synergistic antimicrobial effect. Since different APPs have different biological and chemical stabilities, a targeted formulation strategy should be considered for developing stable and inhalable antimicrobial peptides and proteins. These strategies include the use of sodium chloride to reduce electrostatic interaction between APP and extracellular DNA in sputum, the use of D-enantiomers or dendrimers to minimise protease-mediated degradation and or the use of prodrugs to reduce toxicity. Although great effort has been put towards optimising the biological functions of APPs, studies assessing biological stability in inhalable aerosols are scarce, particularly for novel molecules. As such, formulation and manufacture of inhalable liquid and powder formulations of APPs are underexplored, yet they are crucial areas of research for clinical translation.
中文翻译:

用于吸入治疗的抗菌肽和蛋白质的开发进展
抗菌肽和蛋白质 (APP) 在靶向多重耐药 (MDR) 细菌方面变得越来越重要。APPs 是一个迅速崛起的领域,新分子的生产和进一步优化以增强抗菌功效,同时克服了与生物制剂相关的问题,例如由于半衰期短而导致的潜在毒性和低生物利用度。由于肺部局部药物浓度高,暴露于全身循环的程度较低,因此这些药物的吸入给药可以有效治疗呼吸道感染,从而降低全身毒性。这篇综述描述了最近关于吸入 APP 的研究,包括体外和体内尽可能提供抗菌活性、毒性评估和配方策略。该综述还包括对 APP 与其他抗菌药物联合使用以实现增强的协同抗菌效果的研究。由于不同的 APP 具有不同的生物和化学稳定性,因此应考虑有针对性的制剂策略来开发稳定和可吸入的抗菌肽和蛋白质。这些策略包括使用氯化钠来减少痰液中 APP 和细胞外 DNA 之间的静电相互作用,使用 D-对映异构体或树枝状大分子来最大限度地减少蛋白酶介导的降解和/或使用前药来降低毒性。尽管已经为优化 APP 的生物学功能付出了巨大的努力,但评估可吸入气溶胶生物稳定性的研究却很少,特别是对于新分子。因此,APP 的可吸入液体和粉末制剂的配方和制造尚未得到充分探索,但它们是临床转化研究的关键领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号