当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction of Indole-2-Carboxylates/Carboxylic Acids with Propargylic Alcohols: Dearomative Ring Expansion/Spirocyclization vs Fused Pentacyclics
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-11-19 , DOI: 10.1002/adsc.202101038 K. C. Kumara Swamy 1 , Mallepalli Shankar 2 , Uruvakili Anasuyamma 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-11-19 , DOI: 10.1002/adsc.202101038 K. C. Kumara Swamy 1 , Mallepalli Shankar 2 , Uruvakili Anasuyamma 1
Affiliation
|
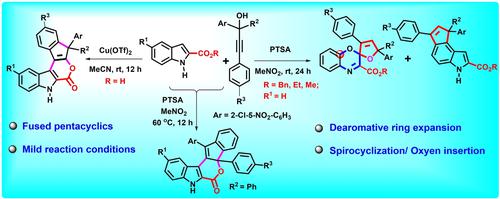
Dearomative ring expansion/spirocyclization of indole-2-carboxylates with propargylic alcohols bearing electron-withdrawing aromatic groups in the presence of PTSA leading to spiro[benzo[b]oxazine-furans] via oxygen insertion along with dihydrocyclopenta[e]indole-2-carboxylates is developed; the same reactants under moderately high temperatures, afford fused pyrano-indolones. In contrast, copper(II) catalyzed annulation of indole-2-carboxylic acids with propargylic alcohols at room temperature provides rapid access to a different class of pentacyclic indene fused pyrano-indolones.
中文翻译:

吲哚-2-羧酸盐/羧酸与炔丙醇的反应:脱环扩环/螺环化与稠合五环
在 PTSA 存在下,吲哚-2-羧酸盐与带有吸电子芳基的炔丙基醇的脱环扩环/螺环化通过氧插入与二氢环戊二烯 [e]indole-2-产生螺[苯并[b]恶嗪-呋喃]开发了羧酸盐;在中等高温下相同的反应物得到稠合的吡喃-吲哚酮。相比之下,铜 (II) 催化的吲哚-2-羧酸与炔丙醇在室温下的环化反应可以快速获得不同类别的五环茚稠合吡喃-吲哚酮。
更新日期:2021-11-19
中文翻译:

吲哚-2-羧酸盐/羧酸与炔丙醇的反应:脱环扩环/螺环化与稠合五环
在 PTSA 存在下,吲哚-2-羧酸盐与带有吸电子芳基的炔丙基醇的脱环扩环/螺环化通过氧插入与二氢环戊二烯 [e]indole-2-产生螺[苯并[b]恶嗪-呋喃]开发了羧酸盐;在中等高温下相同的反应物得到稠合的吡喃-吲哚酮。相比之下,铜 (II) 催化的吲哚-2-羧酸与炔丙醇在室温下的环化反应可以快速获得不同类别的五环茚稠合吡喃-吲哚酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号