当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of Active Catalysts at Work
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-11-19 , DOI: 10.1021/acs.accounts.1c00429 Irene M N Groot 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-11-19 , DOI: 10.1021/acs.accounts.1c00429 Irene M N Groot 1
Affiliation
|
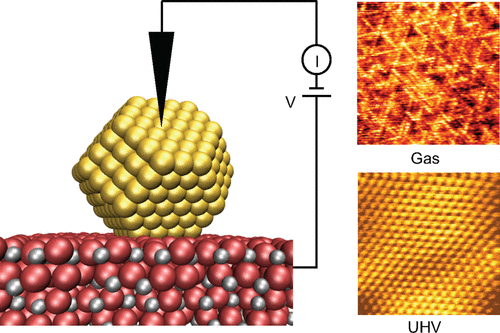
Even after being in business for at least the last 100 years, research into the field of (heterogeneous) catalysis is still vibrant, both in academia and in industry. One of the reasons for this is that around 90% of all chemicals and materials used in everyday life are produced employing catalysis. In 2020, the global catalyst market size reached $35 billion, and it is still steadily increasing every year. Additionally, catalysts will be the driving force behind the transition toward sustainable energy. However, even after having been investigated for 100 years, we still have not reached the holy grail of developing catalysts from rational design instead of from trial-and-error. There are two main reasons for this, indicated by the two so-called “gaps” between (academic) research and actual catalysis. The first one is the “pressure gap”, indicating the 13 orders of magnitude difference in pressure between the ultrahigh vacuum lab conditions and the atmospheric pressures (and higher) of industrial catalysis. The second one is the “materials gap”, indicating the difference in complexity between single-crystal model catalysts of academic research and the real catalysts, consisting of metallic nanoparticles on supports, promoters, fillers, and binders. Although over the past decades significant efforts have been made in closing these gaps, many steps still have to be taken. In this Account, I will discuss the steps we have taken at Leiden University to further our fundamental understanding of heterogeneous catalysis at the (near-)atomic scale. I will focus on bridging the pressure gap, though we are also working on closing the materials gap. Over the past years, we developed state-of-the-art equipment that is able to investigate the (near-)atomic-scale structure of the catalyst surface during the chemical reaction using several surface-science-based techniques such as scanning tunneling microscopy, atomic force microscopy, optical microscopy, and X-ray-based techniques (surface X-ray diffraction, grazing-incidence small-angle X-ray scattering, and X-ray reflectivity, in collaboration with ESRF). Simultaneously with imaging the surface, we can investigate the catalyst’s performance via mass spectrometry, enabling us to link changes in the catalyst structure to its activity, selectivity, or stability. Although we are currently investigating many industrially relevant catalytic systems, I will here focus the discussion on the oxidation of platinum during, for example, CO and NO oxidation, the NO reduction reaction on platinum, and the growth of graphene on liquid (molten) copper. I will show that to be able to obtain the full picture of heterogeneous catalysis, the ability to investigate the catalyst at the (near-)atomic scale during the chemical reaction is a must.
中文翻译:

活性催化剂的研究
即使在过去至少 100 年之后,(多相)催化领域的研究在学术界和工业界仍然充满活力。原因之一是日常生活中使用的所有化学品和材料中约 90% 都是通过催化生产的。 2020年,全球催化剂市场规模达到350亿美元,并且每年还在稳步增长。此外,催化剂将成为向可持续能源转型的驱动力。然而,即使经过100年的研究,我们仍然没有达到通过理性设计而不是通过试错来开发催化剂的圣杯。造成这种情况的主要原因有两个,从(学术)研究和实际催化之间的两个所谓的“差距”可以看出。第一个是“压力差距”,表示超高真空实验室条件与工业催化的大气压力(或更高)之间的压力差异有 13 个数量级。第二个是“材料差距”,表明学术研究的单晶模型催化剂与真实催化剂(由载体、促进剂、填料和粘合剂上的金属纳米粒子组成)之间复杂性的差异。尽管过去几十年来为缩小这些差距做出了重大努力,但仍需采取许多步骤。在这篇文章中,我将讨论我们在莱顿大学为进一步加深我们对(近)原子尺度多相催化的基本理解而采取的步骤。我将专注于缩小压力差距,尽管我们也在努力缩小材料差距。 在过去的几年里,我们开发了最先进的设备,能够使用多种基于表面科学的技术(例如扫描隧道显微镜)研究化学反应过程中催化剂表面的(近)原子尺度结构、原子力显微镜、光学显微镜和基于 X 射线的技术(表面 X 射线衍射、掠入射小角度 X 射线散射和 X 射线反射率,与 ESRF 合作)。在对表面进行成像的同时,我们可以通过质谱研究催化剂的性能,使我们能够将催化剂结构的变化与其活性、选择性或稳定性联系起来。尽管我们目前正在研究许多与工业相关的催化系统,但我在这里将重点讨论铂的氧化,例如CO和NO氧化、铂上的NO还原反应以及液态(熔融)铜上石墨烯的生长。我将证明,为了能够全面了解多相催化,必须具备在化学反应过程中以(近)原子尺度研究催化剂的能力。
更新日期:2021-12-07
中文翻译:

活性催化剂的研究
即使在过去至少 100 年之后,(多相)催化领域的研究在学术界和工业界仍然充满活力。原因之一是日常生活中使用的所有化学品和材料中约 90% 都是通过催化生产的。 2020年,全球催化剂市场规模达到350亿美元,并且每年还在稳步增长。此外,催化剂将成为向可持续能源转型的驱动力。然而,即使经过100年的研究,我们仍然没有达到通过理性设计而不是通过试错来开发催化剂的圣杯。造成这种情况的主要原因有两个,从(学术)研究和实际催化之间的两个所谓的“差距”可以看出。第一个是“压力差距”,表示超高真空实验室条件与工业催化的大气压力(或更高)之间的压力差异有 13 个数量级。第二个是“材料差距”,表明学术研究的单晶模型催化剂与真实催化剂(由载体、促进剂、填料和粘合剂上的金属纳米粒子组成)之间复杂性的差异。尽管过去几十年来为缩小这些差距做出了重大努力,但仍需采取许多步骤。在这篇文章中,我将讨论我们在莱顿大学为进一步加深我们对(近)原子尺度多相催化的基本理解而采取的步骤。我将专注于缩小压力差距,尽管我们也在努力缩小材料差距。 在过去的几年里,我们开发了最先进的设备,能够使用多种基于表面科学的技术(例如扫描隧道显微镜)研究化学反应过程中催化剂表面的(近)原子尺度结构、原子力显微镜、光学显微镜和基于 X 射线的技术(表面 X 射线衍射、掠入射小角度 X 射线散射和 X 射线反射率,与 ESRF 合作)。在对表面进行成像的同时,我们可以通过质谱研究催化剂的性能,使我们能够将催化剂结构的变化与其活性、选择性或稳定性联系起来。尽管我们目前正在研究许多与工业相关的催化系统,但我在这里将重点讨论铂的氧化,例如CO和NO氧化、铂上的NO还原反应以及液态(熔融)铜上石墨烯的生长。我将证明,为了能够全面了解多相催化,必须具备在化学反应过程中以(近)原子尺度研究催化剂的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号