当前位置:
X-MOL 学术
›
Comput. Struct. Biotechnol. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A novel hotspot of gelsolin instability triggers an alternative mechanism of amyloid aggregation
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-11-19 , DOI: 10.1016/j.csbj.2021.11.025 Michela Bollati 1 , Luisa Diomede 2 , Toni Giorgino 1 , Carmina Natale 2 , Elisa Fagnani 1 , Irene Boniardi 1 , Alberto Barbiroli 3 , Rebecca Alemani 1 , Marten Beeg 2 , Marco Gobbi 2 , Ana Fakin 4 , Eloise Mastrangelo 1 , Mario Milani 1 , Gianluca Presciuttini 5 , Edi Gabellieri 5 , Patrizia Cioni 5 , Matteo de Rosa 1
Computational and Structural Biotechnology Journal ( IF 4.4 ) Pub Date : 2021-11-19 , DOI: 10.1016/j.csbj.2021.11.025 Michela Bollati 1 , Luisa Diomede 2 , Toni Giorgino 1 , Carmina Natale 2 , Elisa Fagnani 1 , Irene Boniardi 1 , Alberto Barbiroli 3 , Rebecca Alemani 1 , Marten Beeg 2 , Marco Gobbi 2 , Ana Fakin 4 , Eloise Mastrangelo 1 , Mario Milani 1 , Gianluca Presciuttini 5 , Edi Gabellieri 5 , Patrizia Cioni 5 , Matteo de Rosa 1
Affiliation
|
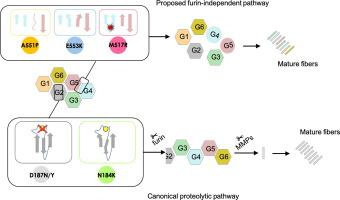
Gelsolin comprises six homologous domains, named G1 to G6. Single point substitutions in this protein are responsible for AGel amyloidosis, a hereditary disease causing progressive corneal lattice dystrophy, cutis laxa, and polyneuropathy. Although several different amyloidogenic variants of gelsolin have been identified, only the most common mutants present in the G2 domain have been thoroughly characterized, leading to clarification of the functional mechanism. The molecular events underlying the pathological aggregation of 3 recently identified mutations, namely A551P, E553K and M517R, all localized at the interface between G4 and G5, are here explored for the first time. Structural studies point to destabilization of the interface between G4 and G5 due to three structural determinants: β-strand breaking, steric hindrance and/or charge repulsion, all implying impairment of interdomain contacts. Such rearrangements decrease the temperature and pressure stability of gelsolin but do not alter its susceptibility to furin cleavage, the first event in the canonical aggregation pathway. These variants also have a greater tendency to aggregate in the unproteolysed forms and exhibit higher proteotoxicity in a -based assay. Our data suggest that aggregation of G4G5 variants follows an alternative, likely proteolysis-independent, pathway.
中文翻译:

凝溶胶蛋白不稳定性的新热点触发淀粉样蛋白聚集的替代机制
凝溶胶蛋白包含六个同源结构域,称为 G1 至 G6。这种蛋白质中的单点替换导致 AGel 淀粉样变性,这是一种导致进行性角膜格子营养不良、皮肤松弛和多发性神经病的遗传性疾病。尽管已经鉴定了凝溶胶蛋白的几种不同的淀粉样变体,但仅对 G2 结构域中最常见的突变体进行了彻底的表征,从而澄清了其功能机制。首次探讨了最近发现的 3 个突变(即 A551P、E553K 和 M517R)病理聚集的分子事件,这些突变均位于 G4 和 G5 之间的界面。结构研究指出,G4 和 G5 之间的界面不稳定是由于三个结构决定因素造成的:β 链断裂、空间位阻和/或电荷排斥,所有这些都意味着域间接触的损害。这种重排降低了凝溶胶蛋白的温度和压力稳定性,但不会改变其对弗林蛋白酶裂解的敏感性,弗林蛋白酶裂解是典型聚集途径中的第一个事件。这些变体还更倾向于以未蛋白水解的形式聚集,并在基于β的测定中表现出更高的蛋白毒性。我们的数据表明,G4G5 变体的聚集遵循一条可能不依赖于蛋白水解的替代途径。
更新日期:2021-11-19
中文翻译:

凝溶胶蛋白不稳定性的新热点触发淀粉样蛋白聚集的替代机制
凝溶胶蛋白包含六个同源结构域,称为 G1 至 G6。这种蛋白质中的单点替换导致 AGel 淀粉样变性,这是一种导致进行性角膜格子营养不良、皮肤松弛和多发性神经病的遗传性疾病。尽管已经鉴定了凝溶胶蛋白的几种不同的淀粉样变体,但仅对 G2 结构域中最常见的突变体进行了彻底的表征,从而澄清了其功能机制。首次探讨了最近发现的 3 个突变(即 A551P、E553K 和 M517R)病理聚集的分子事件,这些突变均位于 G4 和 G5 之间的界面。结构研究指出,G4 和 G5 之间的界面不稳定是由于三个结构决定因素造成的:β 链断裂、空间位阻和/或电荷排斥,所有这些都意味着域间接触的损害。这种重排降低了凝溶胶蛋白的温度和压力稳定性,但不会改变其对弗林蛋白酶裂解的敏感性,弗林蛋白酶裂解是典型聚集途径中的第一个事件。这些变体还更倾向于以未蛋白水解的形式聚集,并在基于β的测定中表现出更高的蛋白毒性。我们的数据表明,G4G5 变体的聚集遵循一条可能不依赖于蛋白水解的替代途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号