当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interrupted CuAAC-Thiolation for the Construction of 1,2,3-Triazole-Fused Eight-Membered Heterocycles from O-/N-Propargyl derived Benzyl Thiosulfonates with Organic Azides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-11-18 , DOI: 10.1002/adsc.202101256 Raju Jannapu Reddy 1 , Waheed Md. 1 , Haritha Kumari Arram 1 , Rama Krishna Gamidi 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-11-18 , DOI: 10.1002/adsc.202101256 Raju Jannapu Reddy 1 , Waheed Md. 1 , Haritha Kumari Arram 1 , Rama Krishna Gamidi 2
Affiliation
|
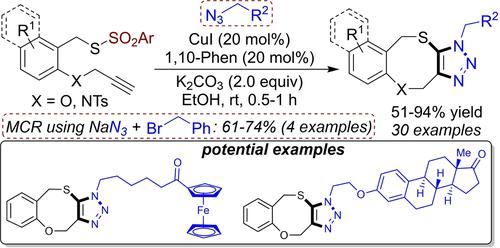
A copper(I)-catalyzed interrupted click-sulfenylation of O-/N-propargyl benzyl thiosulfonates with organic azides has been disclosed. The unified CuAAC-thiolation provides a wide range of triazole-fused eight-membered heterocycles in good to high (51–94%) yields under mild reaction conditions. Moreover, a three-component reaction is also achieved involving O-/N-propargyl benzyl thiosulfonates, benzyl bromide, and sodium azide to deliver fused-triazoles in 61–74% yields. From a synthetic point of view, the present protocol has been demonstrated at gram-scale reactions. A plausible mechanism is also proposed based on experimental results and control experiments.
中文翻译:

用有机叠氮化物从 O-/N-炔丙基衍生的硫代磺酸苄酯构建 1,2,3-三唑-稠合八元杂环的中断 CuAAC-硫醇化
已公开了铜 (I) 催化的O-/N-炔丙基苄基硫代磺酸盐与有机叠氮化物的间断点击亚磺酰化。在温和的反应条件下,统一的 CuAAC-硫醇化提供了范围广泛的三唑稠合八元杂环,产率从好到高(51-94%)。此外,还实现了涉及O-/N-炔丙基苄基硫代磺酸盐、苄基溴和叠氮化钠的三组分反应,以 61-74% 的收率提供稠合三唑。从合成的角度来看,本协议已在克级反应中得到证明。基于实验结果和对照实验,还提出了一种似是而非的机制。
更新日期:2022-01-18
中文翻译:

用有机叠氮化物从 O-/N-炔丙基衍生的硫代磺酸苄酯构建 1,2,3-三唑-稠合八元杂环的中断 CuAAC-硫醇化
已公开了铜 (I) 催化的O-/N-炔丙基苄基硫代磺酸盐与有机叠氮化物的间断点击亚磺酰化。在温和的反应条件下,统一的 CuAAC-硫醇化提供了范围广泛的三唑稠合八元杂环,产率从好到高(51-94%)。此外,还实现了涉及O-/N-炔丙基苄基硫代磺酸盐、苄基溴和叠氮化钠的三组分反应,以 61-74% 的收率提供稠合三唑。从合成的角度来看,本协议已在克级反应中得到证明。基于实验结果和对照实验,还提出了一种似是而非的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号