当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot enantioselective construction of 3,4-dihydro-2H-1,4-oxazines over Ru/Au relay catalysis and its mechanistic serendipity
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-17 , DOI: 10.1039/d1qo01482j Dongfeng Yang 1 , Chengyi Wang 1 , Yu Wang 1 , Guohua Liu 1 , Tanyu Cheng 1 , Rui Liu 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2021-11-17 , DOI: 10.1039/d1qo01482j Dongfeng Yang 1 , Chengyi Wang 1 , Yu Wang 1 , Guohua Liu 1 , Tanyu Cheng 1 , Rui Liu 1
Affiliation
|
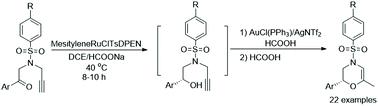
The preparation of enantiomerically pure 1,4-oxazines remains a continuous challenge in synthetic chemistry because of their potential application in the total synthesis of morpholines. Herein, a one-pot asymmetric transfer hydrogenation/cyclization enantio-relay process catalyzed by Ru and Au bimetallic catalysts was developed. This enantio-relay process firstly allows the asymmetric transfer hydrogenation of alkynones to form chiral alkynols in the presence of (S,S)-mesityleneRuClTsDPEN (TsDPEN = N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine) and then the chiral alkynols could be converted into 3,4-dihydro-2H-1,4-oxazines in moderate to good yields with excellent enantioselectivity retention accompanied by AuCl(PPh3)/AgNTf2 and HCOOH. A series of sulfonamide-tethered alkynones were well tolerated in this process. Mechanistic studies indicated that the alkynols formed in the first step were initially transformed into a methylenemorpholine intermediate when AuCl(PPh3)/AgNTf2 and HCOOH were added, which can be further isomerized into chiral 3,4-dihydro-2H-1,4-oxazines with the addition of a second HCOOH. This protocol offers not only a practical method to access 3,4-dihydro-2H-1,4-oxazines from alkynones in an enantioselective version, but also enriches the arsenal of gold chemistry from a mechanistic point of view.
中文翻译:

Ru/Au中继催化3,4-二氢-2H-1,4-恶嗪的一锅对映选择性构建及其机理研究
对映异构纯 1,4-恶嗪的制备仍然是合成化学中的一个持续挑战,因为它们在吗啉的全合成中具有潜在的应用价值。在此,开发了一种由 Ru 和 Au 双金属催化剂催化的一锅不对称转移加氢/环化对映中继工艺。这种对映中继过程首先允许炔酮在 ( S , S ) -均三甲苯RuClTsDPEN (TsDPEN = N -( p -甲苯磺酰基)-1,2-二苯基乙二胺)存在下不对称转移氢化形成手性炔醇,然后是手性炔醇可以转化为 3,4-dihydro-2 H-1,4-恶嗪在中等至良好的产率下具有出色的对映选择性保留,伴随有 AuCl(PPh 3 )/AgNTf 2和 HCOOH。在该过程中,一系列磺酰胺系链炔酮具有良好的耐受性。机理研究表明,当加入AuCl(PPh 3 )/AgNTf 2和HCOOH时,第一步形成的炔醇最初转化为亚甲基吗啉中间体,可进一步异构化为手性3,4-二氢-2 H -1,添加第二个 HCOOH 的 4-恶嗪。该协议不仅提供了一种实用的方法来访问 3,4-dihydro-2 H-1,4-恶嗪以对映选择性形式来自炔酮,但从机械角度来看也丰富了金化学武器库。
更新日期:2021-12-01
中文翻译:

Ru/Au中继催化3,4-二氢-2H-1,4-恶嗪的一锅对映选择性构建及其机理研究
对映异构纯 1,4-恶嗪的制备仍然是合成化学中的一个持续挑战,因为它们在吗啉的全合成中具有潜在的应用价值。在此,开发了一种由 Ru 和 Au 双金属催化剂催化的一锅不对称转移加氢/环化对映中继工艺。这种对映中继过程首先允许炔酮在 ( S , S ) -均三甲苯RuClTsDPEN (TsDPEN = N -( p -甲苯磺酰基)-1,2-二苯基乙二胺)存在下不对称转移氢化形成手性炔醇,然后是手性炔醇可以转化为 3,4-dihydro-2 H-1,4-恶嗪在中等至良好的产率下具有出色的对映选择性保留,伴随有 AuCl(PPh 3 )/AgNTf 2和 HCOOH。在该过程中,一系列磺酰胺系链炔酮具有良好的耐受性。机理研究表明,当加入AuCl(PPh 3 )/AgNTf 2和HCOOH时,第一步形成的炔醇最初转化为亚甲基吗啉中间体,可进一步异构化为手性3,4-二氢-2 H -1,添加第二个 HCOOH 的 4-恶嗪。该协议不仅提供了一种实用的方法来访问 3,4-dihydro-2 H-1,4-恶嗪以对映选择性形式来自炔酮,但从机械角度来看也丰富了金化学武器库。











































 京公网安备 11010802027423号
京公网安备 11010802027423号