当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and biochemical analyses of the tetrameric carboxypeptidase S9Cfn from Fusobacterium nucleatum
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-11-18 , DOI: 10.1107/s2059798321010810 Xin Wang 1 , Meng Ting Cheng 2 , Zhi Peng Chen 2 , Yong Liang Jiang 2 , Yu Shu Ge 2 , Rong Xia 1 , Wen Tao Hou 2
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2021-11-18 , DOI: 10.1107/s2059798321010810 Xin Wang 1 , Meng Ting Cheng 2 , Zhi Peng Chen 2 , Yong Liang Jiang 2 , Yu Shu Ge 2 , Rong Xia 1 , Wen Tao Hou 2
Affiliation
|
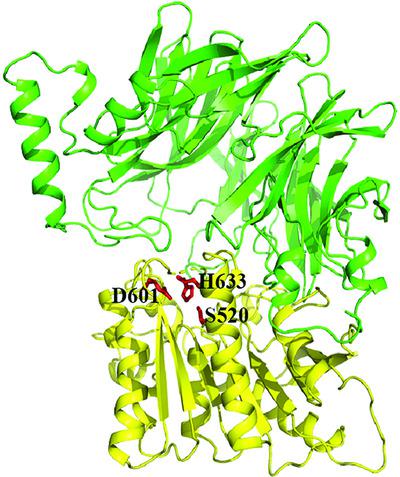
As one of the most abundant bacteria in the human oral cavity, Fusobacterium nucleatum is closely involved in various oral diseases and is also a risk factor for other diseases. The peptidases of F. nucleatum can digest exogenous peptides into amino acids to satisfy its nutrient requirements. Here, a putative F. nucleatum peptidase, termed S9Cfn, which belongs to the S9C peptidase family was identified. Enzymatic activity assays combined with mass-spectrometric analysis revealed that S9Cfn is a carboxypeptidase, but not an aminopeptidase as previously annotated. The crystal structure of the S9Cfn tetramer was solved at 2.6 Å resolution and was found to contain a pair of oligomeric pores in the center. Structural analysis, together with site-directed mutagenesis and enzymatic activity assays, revealed a substrate-entrance tunnel that extends from each oligomeric pore to the catalytic triad, adjacent to which three conserved arginine residues are responsible for substrate binding. Moreover, comparison with other S9 peptidase structures indicated drastic conformational changes of the oligomeric pores during the catalytic cycle. Together, these findings increase the knowledge of this unique type of tetrameric carboxypeptidase and provide insight into the homeostatic control of microbiota in the human oral cavity.
中文翻译:

具核梭杆菌四聚体羧肽酶 S9Cfn 的结构和生化分析
作为人类口腔中含量最多的细菌之一,具核梭杆菌与各种口腔疾病密切相关,也是其他疾病的危险因素。F. nucleatum的肽酶可以将外源肽消化成氨基酸以满足其营养需求。在这里,一个假定的F. nucleatum鉴定了属于 S9C 肽酶家族的肽酶,称为 S9Cfn。酶活性测定与质谱分析相结合表明 S9Cfn 是一种羧肽酶,但不是先前注释的氨肽酶。S9Cfn 四聚体的晶体结构以 2.6 Å 的分辨率解析,发现其中心含有一对低聚孔。结构分析,连同定点诱变和酶活性测定,揭示了从每个寡聚孔延伸到催化三联体的底物入口隧道,三个保守的精氨酸残基与之相邻,负责底物结合。此外,与其他 S9 肽酶结构的比较表明,在催化循环期间寡聚孔的构象发生了剧烈变化。一起,
更新日期:2021-12-06
中文翻译:

具核梭杆菌四聚体羧肽酶 S9Cfn 的结构和生化分析
作为人类口腔中含量最多的细菌之一,具核梭杆菌与各种口腔疾病密切相关,也是其他疾病的危险因素。F. nucleatum的肽酶可以将外源肽消化成氨基酸以满足其营养需求。在这里,一个假定的F. nucleatum鉴定了属于 S9C 肽酶家族的肽酶,称为 S9Cfn。酶活性测定与质谱分析相结合表明 S9Cfn 是一种羧肽酶,但不是先前注释的氨肽酶。S9Cfn 四聚体的晶体结构以 2.6 Å 的分辨率解析,发现其中心含有一对低聚孔。结构分析,连同定点诱变和酶活性测定,揭示了从每个寡聚孔延伸到催化三联体的底物入口隧道,三个保守的精氨酸残基与之相邻,负责底物结合。此外,与其他 S9 肽酶结构的比较表明,在催化循环期间寡聚孔的构象发生了剧烈变化。一起,











































 京公网安备 11010802027423号
京公网安备 11010802027423号