当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequestration of Proteins in Stress Granules Relies on the In-Cell but Not the In Vitro Folding Stability
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-17 , DOI: 10.1021/jacs.1c09589 Nirnay Samanta 1 , Sara S Ribeiro 1 , Mailin Becker 1 , Emeline Laborie 2 , Roland Pollak 1 , Stepan Timr 2, 3 , Fabio Sterpone 2 , Simon Ebbinghaus 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-17 , DOI: 10.1021/jacs.1c09589 Nirnay Samanta 1 , Sara S Ribeiro 1 , Mailin Becker 1 , Emeline Laborie 2 , Roland Pollak 1 , Stepan Timr 2, 3 , Fabio Sterpone 2 , Simon Ebbinghaus 1
Affiliation
|
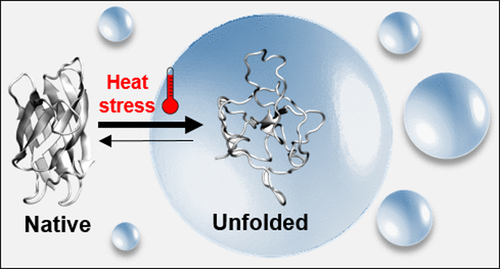
Stress granules (SGs) are among the most studied membraneless organelles that form upon heat stress (HS) to sequester unfolded, misfolded, or aggregated protein, supporting protein quality control (PQC) clearance. The folding states that are primarily associated with SGs, as well as the function of the phase separated environment in adjusting the energy landscapes, remain unknown. Here, we investigate the association of superoxide dismutase 1 (SOD1) proteins with different folding stabilities and aggregation propensities with condensates in cells, in vitro and by simulation. We find that irrespective of aggregation the folding stability determines the association of SOD1 with SGs in cells. In vitro and in silico experiments however suggest that the increased flexibility of the unfolded state constitutes only a minor driving force to associate with the dynamic biomolecular network of the condensate. Specific protein–protein interactions in the cytoplasm in comparison to SGs determine the partitioning of folding states between the respective phases during HS.
中文翻译:

应激颗粒中蛋白质的隔离依赖于细胞内而不是体外折叠稳定性
应力颗粒 (SG) 是研究最多的无膜细胞器之一,它在热应力 (HS) 下形成以隔离未折叠、错误折叠或聚集的蛋白质,支持蛋白质质量控制 (PQC) 清除。主要与 SG 相关的折叠状态,以及相分离环境在调整能量景观中的作用仍然未知。在这里,我们通过体外和模拟研究了具有不同折叠稳定性和聚集倾向的超氧化物歧化酶 1 (SOD1) 蛋白与细胞内冷凝物的关联。我们发现,无论聚合如何,折叠稳定性都决定了 SOD1 与细胞中 SG 的关联。体外和计算机然而,实验表明展开状态的灵活性增加仅构成与凝聚物的动态生物分子网络相关的次要驱动力。与 SG 相比,细胞质中特定的蛋白质-蛋白质相互作用决定了 HS 期间各个阶段之间折叠状态的划分。
更新日期:2021-12-01
中文翻译:

应激颗粒中蛋白质的隔离依赖于细胞内而不是体外折叠稳定性
应力颗粒 (SG) 是研究最多的无膜细胞器之一,它在热应力 (HS) 下形成以隔离未折叠、错误折叠或聚集的蛋白质,支持蛋白质质量控制 (PQC) 清除。主要与 SG 相关的折叠状态,以及相分离环境在调整能量景观中的作用仍然未知。在这里,我们通过体外和模拟研究了具有不同折叠稳定性和聚集倾向的超氧化物歧化酶 1 (SOD1) 蛋白与细胞内冷凝物的关联。我们发现,无论聚合如何,折叠稳定性都决定了 SOD1 与细胞中 SG 的关联。体外和计算机然而,实验表明展开状态的灵活性增加仅构成与凝聚物的动态生物分子网络相关的次要驱动力。与 SG 相比,细胞质中特定的蛋白质-蛋白质相互作用决定了 HS 期间各个阶段之间折叠状态的划分。









































 京公网安备 11010802027423号
京公网安备 11010802027423号