当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Aza-Prins Reaction of 1,2-Dicarbonyl Compounds with 3-Vinyltetrahydroquinolines: Application to the Synthesis of Polycyclic Spirooxindole Derivatives
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-11-18 , DOI: 10.1021/acs.joc.1c01785 Shinichi Saito 1 , Tomohiro Katamura 1 , Rei Tsukazaki 1 , Akito Fujisawa 1 , Yusuke Yoshigoe 1 , Yuichiro Mutoh 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2021-11-18 , DOI: 10.1021/acs.joc.1c01785 Shinichi Saito 1 , Tomohiro Katamura 1 , Rei Tsukazaki 1 , Akito Fujisawa 1 , Yusuke Yoshigoe 1 , Yuichiro Mutoh 1
Affiliation
|
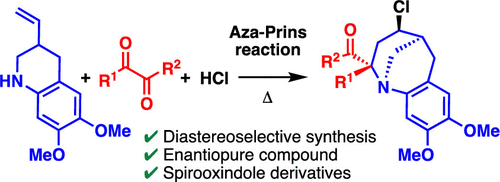
The aza-Prins reaction of 6,7-dimethoxy-3-vinyl-1,2,3,4-tetrahydroquinoline (1) with 1,2-dicarbonyl compounds proceeded smoothly in the presence of HCl, and the corresponding tricyclic benzazocines were isolated in yields of 20–86%. The reaction proceeded in a stereoselective manner, and the formation of the 2,4-trans isomer was observed. The reaction of 1 with an enantiopure ketoester gave the corresponding tricyclic benzazocine as a mixture of diastereomers. The diastereomers were easily separated and converted to enantiopure tricyclic benzazocines. The synthesis of spirooxindole derivatives was achieved by the reaction of 1 with isatin derivatives.
中文翻译:

1,2-二羰基化合物与3-乙烯基四氢喹啉的Aza-Prins反应:在多环螺吲哚衍生物合成中的应用
6,7-二甲氧基-3-乙烯基-1,2,3,4-四氢喹啉( 1 )与1,2-二羰基化合物在HCl存在下的aza-Prins反应顺利进行,并分离出相应的三环苯并佐辛产率为 20-86%。反应以立体选择性方式进行,并观察到2,4-反式异构体的形成。1与对映体纯酮酯反应得到相应的三环苯并佐辛,为非对映异构体的混合物。非对映异构体很容易分离并转化为对映体纯的三环苯并佐辛。螺羟吲哚衍生物的合成是通过1与靛红衍生物的反应来实现的。
更新日期:2021-12-03
中文翻译:

1,2-二羰基化合物与3-乙烯基四氢喹啉的Aza-Prins反应:在多环螺吲哚衍生物合成中的应用
6,7-二甲氧基-3-乙烯基-1,2,3,4-四氢喹啉( 1 )与1,2-二羰基化合物在HCl存在下的aza-Prins反应顺利进行,并分离出相应的三环苯并佐辛产率为 20-86%。反应以立体选择性方式进行,并观察到2,4-反式异构体的形成。1与对映体纯酮酯反应得到相应的三环苯并佐辛,为非对映异构体的混合物。非对映异构体很容易分离并转化为对映体纯的三环苯并佐辛。螺羟吲哚衍生物的合成是通过1与靛红衍生物的反应来实现的。



























 京公网安备 11010802027423号
京公网安备 11010802027423号