Journal of Loss Prevention in the Process Industries ( IF 3.6 ) Pub Date : 2021-11-18 , DOI: 10.1016/j.jlp.2021.104676 Kai Zhang 1 , Wei Gao 1 , Yanchao Li 1 , Zongling Zhang 1 , Sheng Shang 1 , Changshuai Zhang 1 , Xiangfeng Chen 1 , Kai Sun 2
|
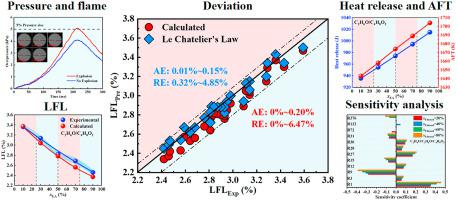
This work is aimed at investigating the lower flammability limits (LFLs) of ethanol, acetone, and ethyl acetate vapor mixtures in air with various mole fractions. The LFLs were measured by a self-made experimental apparatus. Besides, a theoretical calculation model of LFL based on adiabatic constant pressure was established. The heat release, adiabatic flame temperature and the sensitivity of mass burning rate on LFL are analyzed. The results indicated that as the mole fraction of a single substance in a binary mixture or a ternary mixture increases, the LFL changes monotonically. The calculated LFLs are in good agreement with the experiment. The maximum absolute deviation and relative deviation are 0.2% and 6.47% respectively. The trends of heat release and adiabatic flame temperature (AFT) are consistent, meanwhile, the trends of LFL are opposite to them. Besides, the trend of the total peak of heat release rate at LFL is consistent with the heat release. H + O2 < = > O + OH mainly reduces the heat release rate, and CH3 + O < = > CH2O + H mainly increases the heat release rate. The sensitivity coefficients of the maximum main chain branching reaction H + O2 < = > O + OH and the main termination reaction H + O2 (+M) < = > HO2 (+M) of the mass burning rate at the LFL are almost the same.
中文翻译:

乙醇、丙酮和乙酸乙酯蒸气混合物在空气中的燃烧极限较低
这项工作旨在研究空气中不同摩尔分数的乙醇、丙酮和乙酸乙酯蒸气混合物的可燃性下限 (LFL)。LFLs 由自制的实验装置测量。此外,建立了基于绝热恒压的LFL理论计算模型。分析了放热、绝热火焰温度和质量燃烧速率对LFL的敏感性。结果表明,随着二元混合物或三元混合物中单一物质的摩尔分数增加,LFL单调变化。计算出的 LFL 与实验非常吻合。最大绝对偏差和相对偏差分别为0.2%和6.47%。放热趋势与绝热火焰温度(AFT)趋势一致,同时,LFL的趋势与他们相反。此外,LFL 放热率总峰值的趋势与放热一致。氢+氧2 < = > O + OH 主要降低放热率,CH 3 + O < = > CH 2 O + H 主要增加放热率。LFL处质量燃烧速率的最大主链支化反应H + O 2 < = > O + OH和主要终止反应H + O 2 (+M) < = > HO 2 (+M)的灵敏度系数几乎相同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号