Journal of Catalysis ( IF 6.5 ) Pub Date : 2021-11-16 , DOI: 10.1016/j.jcat.2021.11.010 Froze Jameel 1 , Matthias Stein 1
|
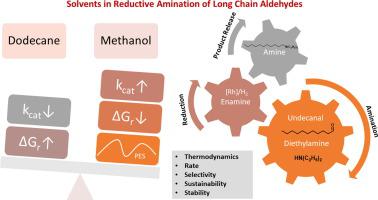
In the global effort to fight climate change, the design and development of novel procedures for complex chemical multi-step reactions is essential for the transformation of chemical industry. The transformation of chemical production processes from petrochemicals towards renewable, sustainable feedstock and the identification of green solvent candidates require a careful assessment of the effects of solvent on thermodynamics and kinetics. As a prime example for the many roles of solvent in chemical catalysis, the reaction mechanism of the homogeneous rhodium-catalyzed reductive amination of 1-undecanal from plant oils with diethylamine forming the long-chain tertiary N,N-diethylundecylamine is investigated. The many roles of solvent during the course of a chemical reactions become apparent when direct substrate-solvent and catalyst-solvent interactions are considered and their polarization effects. Hydrogen bond forming solvent molecules promote the enamine intermediate formation in terms of thermodynamics and kinetics by actively participating as proton transfer agents but a de-solvation penalty for polar groups compromises the overall pathway. The sophisticated bidentate phosphine (SulfoXantPhos)RhH reducing catalyst controls the regioselectivity of the reaction by dedicated ligand-substrate interactions. Its activity is critically dependent on the strength of solvent coordination. The effect of solvent on the reaction rate becomes apparent from a solvent screening of the transition state of the rate-determining step and give a perspective on solvent control of rate constants in this complex multi-step reaction. Only in presence of an appropriate solvent, the calculated Gibbs free potential energy surface becomes shallow and flat and delivers thermodynamic and kinetic parameters in good agreement with experiment.
中文翻译:

溶剂在均相催化中的多种作用 - 还原胺化展示
在全球应对气候变化的努力中,复杂化学多步反应的新程序的设计和开发对于化学工业的转型至关重要。化学生产过程从石化产品向可再生、可持续原料的转变以及绿色溶剂候选物的识别需要仔细评估溶剂对热力学和动力学的影响。作为溶剂在化学催化中的多种作用的一个主要例子,植物油中的 1-十一烷醛与二乙胺形成长链叔N,N的均相铑催化还原胺化反应机理-二乙基十一胺进行了研究。当考虑直接的底物-溶剂和催化剂-溶剂相互作用及其极化效应时,溶剂在化学反应过程中的许多作用变得明显。氢键形成溶剂分子通过作为质子转移剂积极参与,在热力学和动力学方面促进了烯胺中间体的形成,但极性基团的去溶剂化惩罚会损害整个途径。复杂的双齿膦 (SulfoXantPhos)RhH 还原催化剂通过专用的配体-底物相互作用控制反应的区域选择性。它的活性严重依赖于溶剂配位的强度。溶剂对反应速率的影响从决定速率步骤的过渡态的溶剂筛选变得明显,并给出了在这个复杂的多步骤反应中溶剂控制速率常数的观点。只有在合适的溶剂存在下,计算出的吉布斯自由势能面才会变得浅而平坦,并提供与实验非常吻合的热力学和动力学参数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号