当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Modification at Tyrosine with Iminoxyl Radicals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-17 , DOI: 10.1021/jacs.1c09066 Katsuya Maruyama 1 , Takashi Ishiyama 1 , Yohei Seki 1 , Kentaro Sakai 1 , Takaya Togo 1 , Kounosuke Oisaki 1 , Motomu Kanai 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-17 , DOI: 10.1021/jacs.1c09066 Katsuya Maruyama 1 , Takashi Ishiyama 1 , Yohei Seki 1 , Kentaro Sakai 1 , Takaya Togo 1 , Kounosuke Oisaki 1 , Motomu Kanai 1
Affiliation
|
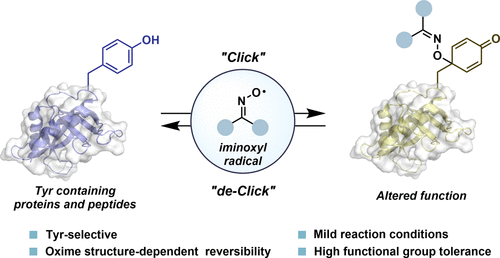
Post-translational modifications (PTMs) of proteins are a biological mechanism for reversibly controlling protein function. Synthetic protein modifications (SPMs) at specific canonical amino acids can mimic PTMs. However, reversible SPMs at hydrophobic amino acid residues in proteins are especially limited. Here, we report a tyrosine (Tyr)-selective SPM utilizing persistent iminoxyl radicals, which are readily generated from sterically hindered oximes via single-electron oxidation. The reactivity of iminoxyl radicals with Tyr was dependent on the steric and electronic demands of oximes; isopropyl methyl piperidinium oxime 1f formed stable adducts, whereas the reaction of tert-butyl methyl piperidinium oxime 1o was reversible. The difference in reversibility between 1f and 1o, differentiated only by one methyl group, is due to the stability of iminoxyl radicals, which is partly dictated by the bond dissociation energy of oxime O–H groups. The Tyr-selective modifications with 1f and 1o proceeded under physiologically relevant, mild conditions. Specifically, the stable Tyr-modification with 1f introduced functional small molecules, including an azobenzene photoswitch, to proteins. Moreover, masking critical Tyr residues by SPM with 1o, and subsequent deconjugation triggered by the treatment with a thiol, enabled on-demand control of protein functions. We applied this reversible Tyr modification with 1o to alter an enzymatic activity and the binding affinity of a monoclonal antibody with an antigen upon modification/deconjugation. The on-demand ON/OFF switch of protein functions through Tyr-selective and reversible covalent-bond formation will provide unique opportunities in biological research and therapeutics.
中文翻译:

用亚氨基自由基修饰酪氨酸的蛋白质
蛋白质的翻译后修饰 (PTM) 是一种可逆地控制蛋白质功能的生物学机制。特定规范氨基酸的合成蛋白修饰 (SPM) 可以模拟 PTM。然而,蛋白质中疏水氨基酸残基上的可逆 SPM 尤其有限。在这里,我们报告了一种酪氨酸 (Tyr) 选择性 SPM,它利用持久的亚氨基氧基自由基,这些自由基很容易通过单电子氧化从空间位阻肟生成。亚氨氧基自由基与 Tyr 的反应性取决于肟的空间和电子需求;异丙基甲基哌啶肟1f形成稳定的加合物,而叔丁基甲基哌啶肟1o的反应是可逆的。1f和1o之间的可逆性差异(仅由一个甲基区分)是由于亚氨基氧基自由基的稳定性,这部分取决于肟 O-H 基团的键解离能。用1f和1o进行的 Tyr 选择性修饰是在生理相关的温和条件下进行的。具体来说,用1f进行的稳定 Tyr 修饰将功能性小分子(包括偶氮苯光开关)引入蛋白质。此外,通过 SPM 用1o掩盖关键的 Tyr 残基,以及随后由硫醇处理引发的解偶联,能够按需控制蛋白质功能。我们用1o应用这种可逆的 Tyr 修饰来改变酶活性和单克隆抗体在修饰/去缀合时与抗原的结合亲和力。通过 Tyr 选择性和可逆共价键形成的蛋白质功能的按需 ON/OFF 开关将为生物学研究和治疗提供独特的机会。
更新日期:2021-12-01
中文翻译:

用亚氨基自由基修饰酪氨酸的蛋白质
蛋白质的翻译后修饰 (PTM) 是一种可逆地控制蛋白质功能的生物学机制。特定规范氨基酸的合成蛋白修饰 (SPM) 可以模拟 PTM。然而,蛋白质中疏水氨基酸残基上的可逆 SPM 尤其有限。在这里,我们报告了一种酪氨酸 (Tyr) 选择性 SPM,它利用持久的亚氨基氧基自由基,这些自由基很容易通过单电子氧化从空间位阻肟生成。亚氨氧基自由基与 Tyr 的反应性取决于肟的空间和电子需求;异丙基甲基哌啶肟1f形成稳定的加合物,而叔丁基甲基哌啶肟1o的反应是可逆的。1f和1o之间的可逆性差异(仅由一个甲基区分)是由于亚氨基氧基自由基的稳定性,这部分取决于肟 O-H 基团的键解离能。用1f和1o进行的 Tyr 选择性修饰是在生理相关的温和条件下进行的。具体来说,用1f进行的稳定 Tyr 修饰将功能性小分子(包括偶氮苯光开关)引入蛋白质。此外,通过 SPM 用1o掩盖关键的 Tyr 残基,以及随后由硫醇处理引发的解偶联,能够按需控制蛋白质功能。我们用1o应用这种可逆的 Tyr 修饰来改变酶活性和单克隆抗体在修饰/去缀合时与抗原的结合亲和力。通过 Tyr 选择性和可逆共价键形成的蛋白质功能的按需 ON/OFF 开关将为生物学研究和治疗提供独特的机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号