当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sterically Stabilized End-On Superoxocopper(II) Complexes and Mechanistic Insights into Their Reactivity with O–H, N–H, and C–H Substrates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-16 , DOI: 10.1021/jacs.1c07837 Sebastian Y Quek 1 , Suman Debnath 1 , Shoba Laxmi 1 , Maurice van Gastel 2 , Tobias Krämer 3, 4 , Jason England 1, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-16 , DOI: 10.1021/jacs.1c07837 Sebastian Y Quek 1 , Suman Debnath 1 , Shoba Laxmi 1 , Maurice van Gastel 2 , Tobias Krämer 3, 4 , Jason England 1, 5
Affiliation
|
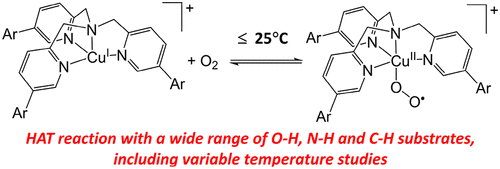
Instability of end-on superoxocopper(II) complexes, with respect to conversion to peroxo-bridged dicopper(II) complexes, has largely constrained their study to very low temperatures. This limits their kinetic capacity to oxidize substrates. In response, we have developed a series of bulky ligands, Ar3-TMPA (Ar = tpb, dpb, dtbpb), and used them to support copper(I) complexes that react with O2 to yield [CuII(η1-O2•–)(Ar3-TMPA)]+ species, which are stable against dimerization at all temperatures. Binding of O2 saturates at subambient temperatures and can be reversed by warming. The onset of oxygenation for the Ar = tpb and dpb systems is observed at 25 °C, and all three [CuII(η1-O2•–)(Ar3-TMPA)]+ complexes are stable against self-decay at temperatures of ≤−20 °C. This provides a wide temperature window for study of these complexes, which was exploited by performing extensive reaction kinetics measurements for [CuII(η1-O2•–)(tpb3-TMPA)]+ using a broad range of O–H, N–H, and C–H bond substrates. This includes correlation of second order rate constants (k2) versus oxidation potentials (Eox) for a range of phenols, construction of Eyring plots, and temperature-dependent kinetic isotope effect (KIE) measurements. The data obtained indicate that reaction with all substrates proceeds via H atom transfer (HAT), reaction with the phenols proceeds with significant charge transfer, and full tunneling of both H and D atoms occurs in the case of 1,2-diphenylhydrazine and 4-methoxy-2,6-di-tert-butylphenol. Oxidation of C–H bonds proved to be kinetically challenging, and whereas [CuII(η1-O2•–)(tpb3-TMPA)]+ can oxidize moderately strong O–H and N–H bonds, it is only able to oxidize very weak C–H bonds.
中文翻译:

空间稳定的末端超氧铜 (II) 配合物及其与 O-H、N-H 和 C-H 底物反应性的机理研究
末端超氧铜 (II) 配合物在转化为过氧桥联二铜 (II) 配合物方面的不稳定性在很大程度上限制了他们在极低温度下的研究。这限制了它们氧化底物的动力学能力。作为回应,我们开发了一系列庞大的配体 Ar 3 -TMPA (Ar = tpb, dpb, dtbpb),并使用它们来支持与 O 2反应生成[Cu II (η 1 - O 2 •– )(Ar 3 -TMPA)] +物质,在所有温度下对二聚化都是稳定的。O 2的结合在低于环境温度时饱和,并且可以通过变暖来逆转。Ar = tpb 和 dpb 系统在 25 °C 时开始氧化,所有三种 [Cu II (η 1 -O 2 •– )(Ar 3 -TMPA)] +配合物在温度≤−20 °C。这为研究这些配合物提供了一个较宽的温度窗口,通过使用广泛的 O-H对 [Cu II (η 1 -O 2 •– )(tpb 3 -TMPA)] +进行广泛的反应动力学测量来利用这一点、N-H 和 C-H 键合底物。这包括二阶速率常数的相关性(k 2 ) 与一系列苯酚的氧化电位 ( E ox )、Eyring 图的构建和温度相关的动力学同位素效应 (KIE) 测量。获得的数据表明,与所有底物的反应通过 H 原子转移 (HAT) 进行,与苯酚的反应进行显着的电荷转移,并且在 1,2-二苯肼和 4-甲氧基-2,6-二叔丁基苯酚。C-H 键的氧化被证明在动力学上具有挑战性,而 [Cu II (η 1 -O 2 •– )(tpb 3 -TMPA)] + 可以氧化中等强度的 O-H 和 N-H 键,它只能氧化非常弱的 C-H 键。
更新日期:2021-12-01
中文翻译:

空间稳定的末端超氧铜 (II) 配合物及其与 O-H、N-H 和 C-H 底物反应性的机理研究
末端超氧铜 (II) 配合物在转化为过氧桥联二铜 (II) 配合物方面的不稳定性在很大程度上限制了他们在极低温度下的研究。这限制了它们氧化底物的动力学能力。作为回应,我们开发了一系列庞大的配体 Ar 3 -TMPA (Ar = tpb, dpb, dtbpb),并使用它们来支持与 O 2反应生成[Cu II (η 1 - O 2 •– )(Ar 3 -TMPA)] +物质,在所有温度下对二聚化都是稳定的。O 2的结合在低于环境温度时饱和,并且可以通过变暖来逆转。Ar = tpb 和 dpb 系统在 25 °C 时开始氧化,所有三种 [Cu II (η 1 -O 2 •– )(Ar 3 -TMPA)] +配合物在温度≤−20 °C。这为研究这些配合物提供了一个较宽的温度窗口,通过使用广泛的 O-H对 [Cu II (η 1 -O 2 •– )(tpb 3 -TMPA)] +进行广泛的反应动力学测量来利用这一点、N-H 和 C-H 键合底物。这包括二阶速率常数的相关性(k 2 ) 与一系列苯酚的氧化电位 ( E ox )、Eyring 图的构建和温度相关的动力学同位素效应 (KIE) 测量。获得的数据表明,与所有底物的反应通过 H 原子转移 (HAT) 进行,与苯酚的反应进行显着的电荷转移,并且在 1,2-二苯肼和 4-甲氧基-2,6-二叔丁基苯酚。C-H 键的氧化被证明在动力学上具有挑战性,而 [Cu II (η 1 -O 2 •– )(tpb 3 -TMPA)] + 可以氧化中等强度的 O-H 和 N-H 键,它只能氧化非常弱的 C-H 键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号