当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biopatterned Reorganization of Alkaloids Enabled by Ring-Opening Functionalization of Tertiary Amines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-16 , DOI: 10.1021/jacs.1c10205 Hyeonggeun Lim 1 , Sikwang Seong 1 , Youyoung Kim 1, 2 , Sangwon Seo 1, 2 , Sunkyu Han 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-11-16 , DOI: 10.1021/jacs.1c10205 Hyeonggeun Lim 1 , Sikwang Seong 1 , Youyoung Kim 1, 2 , Sangwon Seo 1, 2 , Sunkyu Han 1, 2
Affiliation
|
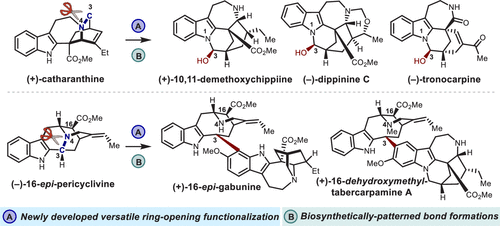
Biosynthetic processes often involve reorganization of one family of natural products to another. Chemical emulation of nature’s rearrangement-based structural diversification strategy would enable the conversion of readily available natural products to other value-added secondary metabolites. However, the development of a chemical method that can be universally applied to structurally diverse natural products is nontrivial. Key to the successful reorganization of complex molecules is a versatile and mild bond-cleaving method that correctly places desired functionality, facilitating the target synthesis. Here, we report a ring-opening functionalization of a tertiary amine that can introduce desired functionalities in the context of alkaloids reorganization. The semistability of the difluoromethylated ammonium salt, accessed by the reaction of tertiary amine and in situ generated difluorocarbene, enabled the attack at the α-position by various external nucleophiles. The utility and generality of the method is highlighted by its applications in the transformation of securinega, iboga, and sarpagine alkaloids to neosecurinega, chippiine/dippinine, and vobasine-type bisindole alkaloids, respectively. During the course of these biosynthetically inspired reorganizations, we could explore chemical reactivities of biogenetically relevant precursors.
中文翻译:

通过叔胺的开环功能化实现生物碱的生物模式重组
生物合成过程通常涉及将一个天然产物家族重组为另一个家族。对自然基于重排的结构多样化策略的化学模拟将使现成的天然产物转化为其他增值次级代谢物。然而,开发一种可普遍应用于结构多样的天然产物的化学方法并非易事。复杂分子成功重组的关键是一种通用且温和的键断裂方法,该方法可以正确放置所需的功能,促进目标合成。在这里,我们报告了叔胺的开环功能化,可以在生物碱重组的背景下引入所需的功能。二氟甲基化铵盐的半稳定性,原位产生二氟卡宾,使各种外部亲核试剂能够攻击α位。该方法的实用性和通用性突出体现在其在将 securinega、iboga 和沙帕金生物碱分别转化为 neosecurinega、chippiine/dippinine 和 vobasine 型双吲哚生物碱中的应用。在这些受生物合成启发的重组过程中,我们可以探索生物遗传相关前体的化学反应性。
更新日期:2021-12-01
中文翻译:

通过叔胺的开环功能化实现生物碱的生物模式重组
生物合成过程通常涉及将一个天然产物家族重组为另一个家族。对自然基于重排的结构多样化策略的化学模拟将使现成的天然产物转化为其他增值次级代谢物。然而,开发一种可普遍应用于结构多样的天然产物的化学方法并非易事。复杂分子成功重组的关键是一种通用且温和的键断裂方法,该方法可以正确放置所需的功能,促进目标合成。在这里,我们报告了叔胺的开环功能化,可以在生物碱重组的背景下引入所需的功能。二氟甲基化铵盐的半稳定性,原位产生二氟卡宾,使各种外部亲核试剂能够攻击α位。该方法的实用性和通用性突出体现在其在将 securinega、iboga 和沙帕金生物碱分别转化为 neosecurinega、chippiine/dippinine 和 vobasine 型双吲哚生物碱中的应用。在这些受生物合成启发的重组过程中,我们可以探索生物遗传相关前体的化学反应性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号