当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coincorporation of N and S into Zero-Valent Iron to Enhance TCE Dechlorination: Kinetics, Electron Efficiency, and Dechlorination Capacity
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-11-17 , DOI: 10.1021/acs.est.1c03784 Li Gong 1 , Xiaojiang Qiu 1 , Dong Cheng 1 , Yao Hu 1 , Zaizhi Zhang 1 , Qunsen Yuan 1 , Dezhi Yang 1 , Chengshuai Liu 2 , Liyuan Liang 3 , Feng He 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2021-11-17 , DOI: 10.1021/acs.est.1c03784 Li Gong 1 , Xiaojiang Qiu 1 , Dong Cheng 1 , Yao Hu 1 , Zaizhi Zhang 1 , Qunsen Yuan 1 , Dezhi Yang 1 , Chengshuai Liu 2 , Liyuan Liang 3 , Feng He 1
Affiliation
|
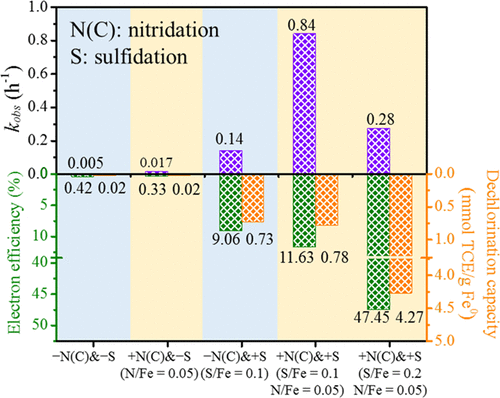
Sulfidated zero-valent iron (S-ZVI) enhances the degradation of chlorinated hydrocarbon (CHC) in contaminated groundwater. Despite numerous studies of S-ZVI, a versatile strategy to improve its dechlorination kinetics, electron efficiency (εe), and dechlorination capacity is still needed. Here, we used heteroatom incorporation of N(C) and S by ball-milling of microscale ZVI with melamine and sulfur via nitridation and sulfidation to synthesize S-N(C)-mZVIbm particles that contain reactive Fe-NX(C) and FeS species. Sulfidation and nitridation synergistically increased the trichloroethene (TCE) dechlorination rate, with reaction constants kSA of 2.98 × 10–2 L·h–1·m–2 by S-N(C)-mZVIbm, compared to 1.77 × 10–3 and 8.15 × 10–5 L·h–1·m–2 by S-mZVIbm and N(C)-mZVIbm, respectively. Data show that sulfidation suppressed the reductive dissociation of N(C) from S-N(C)-mZVIbm, which stabilized the reactive Fe-NX(C) and reserved electrons for TCE dechlorination. In addition to lowering H2 production, S-N(C)-mZVIbm dechlorinated TCE to less reduced products (e.g., acetylene), contributing to the material’s higher εe and dechlorination capacity. This synergistic effect on TCE degradation can be extended to other recalcitrant CHCs (e.g., chloroform) in both deionized and groundwater. This multiheteroatom incorporation approach to optimize ZVI for groundwater remediation provides a basis for further advances in reactive material synthesis.
中文翻译:

将 N 和 S 并入零价铁以增强 TCE 脱氯:动力学、电子效率和脱氯能力
硫化零价铁 (S-ZVI) 会促进受污染地下水中氯化烃 (CHC) 的降解。尽管对 S-ZVI 进行了大量研究,但仍需要一种通用策略来提高其脱氯动力学、电子效率 (ε e ) 和脱氯能力。在这里,我们通过氮化和硫化,通过微尺度 ZVI 与三聚氰胺和硫的球磨,使用 N(C) 和 S 的杂原子掺入来合成含有反应性 Fe-N X (C) 和 FeS 的SN(C)-mZVI bm颗粒物种。硫化和氮化协同提高了三氯乙烯 (TCE) 的脱氯率,反应常数k SA为 2.98 × 10 –2 L·h –1 ·m–2由 SN(C)-mZVI bm,与 1.77 × 10 –3和 8.15 × 10 –5 L·h –1 ·m –2由 S-mZVI bm和 N(C)-mZVI bm分别相比。数据显示硫化抑制了 N(C) 从 SN(C)-mZVI bm的还原解离,这稳定了反应性 Fe-N X (C) 和保留电子用于 TCE 脱氯。除了降低 H 2产量外,SN(C)-mZVI bm 将TCE 脱氯为还原较少的产物(例如乙炔),有助于材料的更高 ε e和脱氯能力。这种对 TCE 降解的协同效应可以扩展到去离子水和地下水中的其他顽固性 CHC(例如氯仿)。这种为地下水修复优化 ZVI 的多杂原子掺入方法为反应性材料合成的进一步进展奠定了基础。
更新日期:2021-12-07
中文翻译:

将 N 和 S 并入零价铁以增强 TCE 脱氯:动力学、电子效率和脱氯能力
硫化零价铁 (S-ZVI) 会促进受污染地下水中氯化烃 (CHC) 的降解。尽管对 S-ZVI 进行了大量研究,但仍需要一种通用策略来提高其脱氯动力学、电子效率 (ε e ) 和脱氯能力。在这里,我们通过氮化和硫化,通过微尺度 ZVI 与三聚氰胺和硫的球磨,使用 N(C) 和 S 的杂原子掺入来合成含有反应性 Fe-N X (C) 和 FeS 的SN(C)-mZVI bm颗粒物种。硫化和氮化协同提高了三氯乙烯 (TCE) 的脱氯率,反应常数k SA为 2.98 × 10 –2 L·h –1 ·m–2由 SN(C)-mZVI bm,与 1.77 × 10 –3和 8.15 × 10 –5 L·h –1 ·m –2由 S-mZVI bm和 N(C)-mZVI bm分别相比。数据显示硫化抑制了 N(C) 从 SN(C)-mZVI bm的还原解离,这稳定了反应性 Fe-N X (C) 和保留电子用于 TCE 脱氯。除了降低 H 2产量外,SN(C)-mZVI bm 将TCE 脱氯为还原较少的产物(例如乙炔),有助于材料的更高 ε e和脱氯能力。这种对 TCE 降解的协同效应可以扩展到去离子水和地下水中的其他顽固性 CHC(例如氯仿)。这种为地下水修复优化 ZVI 的多杂原子掺入方法为反应性材料合成的进一步进展奠定了基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号