Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2021-11-17 , DOI: 10.1016/j.apcatb.2021.120931 Laura Collado 1, 2 , Patricia Reñones 1 , Javier Fermoso 3 , Fernando Fresno 1 , Leoncio Garrido 4 , Virginia Pérez-Dieste 5 , Carlos Escudero 5 , María D. Hernández-Alonso 6 , Juan M. Coronado 3 , David P. Serrano 2, 3 , Víctor A. de la Peña O’Shea 1
|
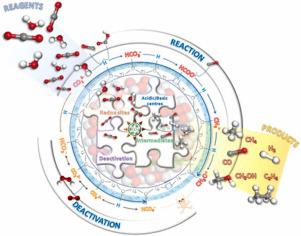
The development of sustainable processes for CO2 reduction to fuels and chemicals is one of the most important challenges to provide clean energy solutions. The use of sunlight as renewable energy source is an interesting alternative to power the electron transfer required for artificial photosynthesis. Even if redox sites are mainly responsible for this process, other reactive acidic/basic centers also contribute to the overall reaction pathway. However, a full understanding of the CO2 photoreduction mechanism is still a scientific challenge. In fact, the lack of agreement on standardized comparison criteria leads to a wide distribution of reported productions, even using the same catalyst, which hinders a reliable interpretation. An additional difficulty is ascertaining the origin of carbon-containing products and effect of surface carbon residues, as well as the reaction intermediates and products under real dynamic conditions. To determine the elusive reaction mechanism, we report an interconnected strategy combining in-situ spectroscopies, theoretical studies and catalytic experiments. These studies show that CO2 photoreduction productions are influenced by the presence of carbon deposits (i.e. organic molecules, carbonates and bicarbonates) over the TiO2 surface. Most importantly, the acid/base character of the surface and the reaction medium play a key role in the selectivity and deactivation pathways. This TiO2 deactivation is mainly initiated by the formation of carbonates and peroxo- species, while activity can be partially recovered by a mild acid washing treatment. We anticipate that these findings and methodology enlighten the main shadows still covering the CO2 reduction mechanism, and, most importantly, provide essential clues for the design of emergent materials and reactions for photo(electro)catalytic energy conversion.
中文翻译:

TiO2 表面酸性/碱性中心和氧化还原位点在光催化 CO2 还原中的作用
开发将 CO 2还原为燃料和化学品的可持续工艺是提供清洁能源解决方案的最重要挑战之一。使用阳光作为可再生能源是一种有趣的替代方案,可以为人工光合作用所需的电子转移提供动力。即使氧化还原位点主要负责该过程,其他反应性酸性/碱性中心也有助于整个反应途径。然而,充分了解 CO 2光还原机制仍然是一个科学挑战。事实上,标准化的比较标准缺乏一致导致报告的生产分布广泛,即使使用相同的催化剂,这阻碍了可靠的解释。另一个困难是确定含碳产物的来源和表面碳残留的影响,以及真实动态条件下的反应中间体和产物。为了确定难以捉摸的反应机制,我们报告了一种结合原位光谱、理论研究和催化实验的相互关联的策略。这些研究表明 CO 2 光还原产物受 TiO 2 上的碳沉积物(即有机分子、碳酸盐和碳酸氢盐)的影响表面。最重要的是,表面和反应介质的酸/碱特性在选择性和失活途径中起着关键作用。这种 TiO 2失活主要是由碳酸盐和过氧物质的形成引发的,而活性可以通过温和的酸洗处理部分恢复。我们预计这些发现和方法将揭示仍然涵盖 CO 2还原机制的主要阴影,最重要的是,为光(电)催化能量转换的新兴材料和反应的设计提供基本线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号