Journal of Hepatology ( IF 25.7 ) Pub Date : 2021-11-15 , DOI: 10.1016/j.jhep.2021.10.029 Mary E Rinella 1 , Jean-Francois Dufour 2 , Quentin M Anstee 3 , Zachary Goodman 4 , Zobair Younossi 4 , Stephen A Harrison 5 , Rohit Loomba 6 , Arun J Sanyal 7 , Martin Bonacci 8 , Aldo Trylesinski 8 , Macky Natha 8 , Reshma Shringarpure 8 , Tanya Granston 8 , Aditya Venugopal 8 , Vlad Ratziu 9
|
Background & Aims
Non-alcoholic steatohepatitis (NASH) is a chronic, progressive fibrotic liver disease that can lead to cirrhosis. While liver biopsy is considered the reference standard for the histologic diagnosis of NASH and staging of fibrosis, its use in clinical practice is limited. Non-invasive tests (NITs) are increasingly being used to identify and stage liver fibrosis in patients with NASH, and several can assess liver-related outcomes. We report changes in various NITs in patients treated with obeticholic acid (OCA) or placebo in the phase III REGENERATE study.
Methods
Patients with NASH and fibrosis stage F2 or F3 (n = 931) were randomized (1:1:1) to receive placebo, OCA 10 mg, or OCA 25 mg once daily. Various NITs based on clinical chemistry and/or imaging were evaluated at baseline and throughout the study.
Results
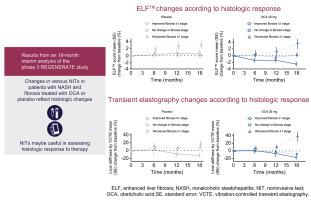
Rapid, sustained reductions from baseline in alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyltransferase levels, as well as in Fibrosis-4 (FIB-4), FibroTest, FibroMeter, and FibroScan-AST scores were observed in OCA-treated vs. placebo-treated patients. Reduction in liver stiffness by vibration-controlled transient elastography was observed in the OCA 25 mg group vs. the placebo group at Month 18. NIT changes were associated with shifts in histologic fibrosis stage. The greatest improvements were observed in patients with ≥1-stage fibrosis improvement; however, improvements in ALT, AST, FIB-4, and FibroTest were also observed in OCA-treated patients whose histologic fibrosis remained stable.
Conclusions
Based on the REGENERATE Month 18 interim analysis, rapid and sustained improvements in various NITs were observed with OCA treatment. Dynamic changes in selected NITs separated histologic responders from non-responders. These results suggest that NITs may be useful in assessing histologic response to OCA therapy.
ClinicalTrials.gov number
NCT02548351
Lay summary
Non-alcoholic steatohepatitis (NASH) is a chronic, progressive liver disease that can lead to cirrhosis. To diagnose and assess liver fibrosis (scarring) in patients with NASH, non-invasive tests (NITs) are increasingly being used rather than liver biopsy, which is invasive, expensive, and can be risky. In the REGENERATE study, which is evaluating the effects of obeticholic acid vs. placebo in patients with NASH, various NITs were also evaluated. This analysis shows that improvements in levels of certain blood components, as well as favorable results of ultrasound imaging and proprietary tests of liver function, were associated with improvements in liver fibrosis after treatment with obeticholic acid, suggesting that NITs may be useful alternatives to liver biopsy in assessing NASH patients’ response to therapy.
中文翻译:

NASH 患者对奥贝胆酸反应的无创评估:REGENERATE 研究的结果
背景与目标
非酒精性脂肪性肝炎 (NASH) 是一种慢性进行性纤维化肝病,可导致肝硬化。虽然肝活检被认为是 NASH 组织学诊断和纤维化分期的参考标准,但其在临床实践中的应用受到限制。非侵入性检测 (NIT) 越来越多地用于识别和分期 NASH 患者的肝纤维化,其中一些可以评估与肝脏相关的结果。我们报告了在 III 期 REGENERATE 研究中接受奥贝胆酸 (OCA) 或安慰剂治疗的患者的各种 NIT 的变化。
方法
患有 NASH 和纤维化分期 F2 或 F3 的患者 (n = 931) 被随机 (1:1:1) 接受安慰剂、OCA 10 mg 或 OCA 25 mg,每天一次。在基线和整个研究过程中评估了基于临床化学和/或成像的各种 NIT。
结果
在丙氨酸氨基转移酶 (ALT)、天冬氨酸氨基转移酶 (AST) 和γ-谷氨酰转移酶水平以及 Fibrosis-4 (FIB-4)、FibroTest、FibroMeter 和 FibroScan-AST 评分中观察到从基线快速持续降低OCA 处理的对比 安慰剂治疗的患者。在第 18 个月时,OCA 25 mg 组与安慰剂组相比观察到通过振动控制的瞬时弹性成像降低肝脏硬度。NIT 变化与组织学纤维化阶段的变化相关。在 ≥ 1 期纤维化改善的患者中观察到最大的改善;然而,在组织学纤维化保持稳定的 OCA 治疗患者中也观察到 ALT、AST、FIB-4 和 FibroTest 的改善。
结论
根据 REGENERATE 第 18 个月的中期分析,OCA 治疗观察到各种 NIT 的快速和持续改善。选定 NIT 的动态变化将组织学反应者与无反应者区分开来。这些结果表明 NIT 可能有助于评估对 OCA 治疗的组织学反应。
ClinicalTrials.gov 编号
NCT02548351
外行总结
非酒精性脂肪性肝炎 (NASH) 是一种慢性进行性肝病,可导致肝硬化。为了诊断和评估 NASH 患者的肝纤维化(瘢痕形成),越来越多地使用非侵入性检测 (NIT),而不是肝活检,后者具有侵入性、昂贵且可能存在风险。在 REGENERATE 研究中,该研究正在评估奥贝胆酸与. 除了在 NASH 患者中使用安慰剂外,还评估了各种 NIT。该分析表明,某些血液成分水平的改善,以及超声成像和肝功能专有测试的有利结果,与奥贝胆酸治疗后肝纤维化的改善有关,表明 NIT 可能是肝活检的有用替代品评估 NASH 患者对治疗的反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号