当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxidative Metabolism as a Modulator of Kratom’s Biological Actions
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-11-16 , DOI: 10.1021/acs.jmedchem.1c01111 Soumen Chakraborty 1 , Rajendra Uprety 2 , Samuel T Slocum 3 , Takeshi Irie 4 , Valerie Le Rouzic 2 , Xiaohai Li 5 , Lisa L Wilson 6 , Brittany Scouller 7 , Amy F Alder 7 , Andrew C Kruegel 8 , Michael Ansonoff 9 , Andras Varadi 2 , Shainnel O Eans 6 , Amanda Hunkele 2 , Abdullah Allaoa 2 , Sanjay Kalra 2 , Jin Xu 2 , Ying Xian Pan 2 , John Pintar 9 , Bronwyn M Kivell 7 , Gavril W Pasternak 2 , Michael D Cameron 5 , Jay P McLaughlin 6 , Dalibor Sames 8 , Susruta Majumdar 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2021-11-16 , DOI: 10.1021/acs.jmedchem.1c01111 Soumen Chakraborty 1 , Rajendra Uprety 2 , Samuel T Slocum 3 , Takeshi Irie 4 , Valerie Le Rouzic 2 , Xiaohai Li 5 , Lisa L Wilson 6 , Brittany Scouller 7 , Amy F Alder 7 , Andrew C Kruegel 8 , Michael Ansonoff 9 , Andras Varadi 2 , Shainnel O Eans 6 , Amanda Hunkele 2 , Abdullah Allaoa 2 , Sanjay Kalra 2 , Jin Xu 2 , Ying Xian Pan 2 , John Pintar 9 , Bronwyn M Kivell 7 , Gavril W Pasternak 2 , Michael D Cameron 5 , Jay P McLaughlin 6 , Dalibor Sames 8 , Susruta Majumdar 1
Affiliation
|
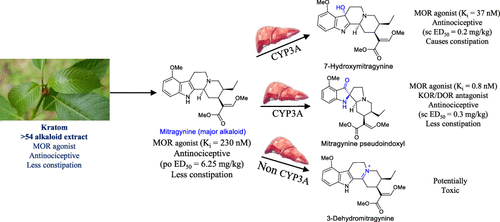
The leaves of Mitragyna speciosa (kratom), a plant native to Southeast Asia, are increasingly used as a pain reliever and for attenuation of opioid withdrawal symptoms. Using the tools of natural products chemistry, chemical synthesis, and pharmacology, we provide a detailed in vitro and in vivo pharmacological characterization of the alkaloids in kratom. We report that metabolism of kratom’s major alkaloid, mitragynine, in mice leads to formation of (a) a potent mu opioid receptor agonist antinociceptive agent, 7-hydroxymitragynine, through a CYP3A-mediated pathway, which exhibits reinforcing properties, inhibition of gastrointestinal (GI) transit and reduced hyperlocomotion, (b) a multifunctional mu agonist/delta-kappa antagonist, mitragynine pseudoindoxyl, through a CYP3A-mediated skeletal rearrangement, displaying reduced hyperlocomotion, inhibition of GI transit and reinforcing properties, and (c) a potentially toxic metabolite, 3-dehydromitragynine, through a non-CYP oxidation pathway. Our results indicate that the oxidative metabolism of the mitragynine template beyond 7-hydroxymitragynine may have implications in its overall pharmacology in vivo.
中文翻译:

氧化代谢作为 Kratom 生物作用的调节剂
Mitragyna speciosa (kratom)的叶子是一种原产于东南亚的植物,越来越多地用作止痛药和减轻阿片类药物戒断症状。使用天然产物化学、化学合成和药理学的工具,我们提供了详细的体外和体内kratom 中生物碱的药理学特性。我们报告说,kratom 的主要生物碱帽柱木碱在小鼠体内的代谢导致形成 (a) 一种有效的 μ 阿片受体激动剂镇痛剂 7-羟基帽柱木碱,通过 CYP3A 介导的途径,它表现出增强特性,抑制胃肠道 (GI) ) 运输和减少过度运动,(b) 多功能 μ 激动剂/δ-κ 拮抗剂,帽柱木碱假吲哚,通过 CYP3A 介导的骨骼重排,显示减少过度运动,抑制 GI 运输和强化特性,和 (c) 潜在毒性代谢物, 3-脱氢帽柱木碱,通过非 CYP 氧化途径。体内。
更新日期:2021-11-25
中文翻译:

氧化代谢作为 Kratom 生物作用的调节剂
Mitragyna speciosa (kratom)的叶子是一种原产于东南亚的植物,越来越多地用作止痛药和减轻阿片类药物戒断症状。使用天然产物化学、化学合成和药理学的工具,我们提供了详细的体外和体内kratom 中生物碱的药理学特性。我们报告说,kratom 的主要生物碱帽柱木碱在小鼠体内的代谢导致形成 (a) 一种有效的 μ 阿片受体激动剂镇痛剂 7-羟基帽柱木碱,通过 CYP3A 介导的途径,它表现出增强特性,抑制胃肠道 (GI) ) 运输和减少过度运动,(b) 多功能 μ 激动剂/δ-κ 拮抗剂,帽柱木碱假吲哚,通过 CYP3A 介导的骨骼重排,显示减少过度运动,抑制 GI 运输和强化特性,和 (c) 潜在毒性代谢物, 3-脱氢帽柱木碱,通过非 CYP 氧化途径。体内。











































 京公网安备 11010802027423号
京公网安备 11010802027423号