当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective [3 + 2] Cycloaddition of Quinoxalin-2(1H)-ones with Donor–Acceptor Cyclopropanes: Efficient Synthesis of Tetrahydro pyrrolo[1,2-a]quinoxalin-4(5H)-ones
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-15 , DOI: 10.1021/acs.joc.1c01872 Himangsu Sekhar Dutta 1 , Ashfaq Ahmad 2 , Afsar Ali Khan 1 , Mohit Kumar 1 , Raziullah 2 , Jayanti Vaishnav 3 , Manoj Gangwar 4 , Ravi Sankar Ampapathi 3 , Dipankar Koley 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2021-11-15 , DOI: 10.1021/acs.joc.1c01872 Himangsu Sekhar Dutta 1 , Ashfaq Ahmad 2 , Afsar Ali Khan 1 , Mohit Kumar 1 , Raziullah 2 , Jayanti Vaishnav 3 , Manoj Gangwar 4 , Ravi Sankar Ampapathi 3 , Dipankar Koley 1, 2
Affiliation
|
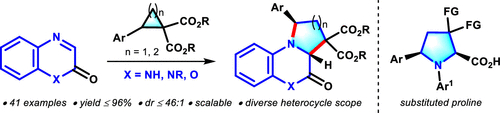
A ytterbium triflate-catalyzed diastereoselective [3 + 2] cycloaddition of quinoxalinones with donor–acceptor cyclopropanes and cyclobutanes is described. A series of tetrahydropyrrolo-quinoxalinone derivatives were obtained in high yields (up to 96%) with excellent diastereoselectivities (up to 46:1). Other medicinally important heterocycles like benzoxazinone, isoquinoxalinone, and dibenzoxazepine derivatives were also suitable for the desired annulation reaction. The current method is applicable for the scale-up reaction. Further, the utility of this annulation reaction is demonstrated by the synthesis of densely functionalized proline derivatives.
中文翻译:

Quinoxalin-2(1H)-ones 与供体-受体环丙烷的非对映选择性 [3 + 2] 环加成:四氢吡咯并[1,2-a]quinoxalin-4(5H)-ones 的高效合成
描述了三氟甲磺酸镱催化的喹喔啉酮与供体-受体环丙烷和环丁烷的非对映选择性 [3 + 2] 环加成反应。以高产率(高达 96%)获得一系列四氢吡咯并喹喔啉酮衍生物,并具有优异的非对映选择性(高达 46:1)。其他药用上重要的杂环化合物,如苯并恶嗪酮、异喹喔啉酮和二苯并恶唑衍生物,也适用于所需的环化反应。本方法适用于放大反应。此外,这种环化反应的效用通过密集官能化脯氨酸衍生物的合成得到证实。
更新日期:2021-12-03
中文翻译:

Quinoxalin-2(1H)-ones 与供体-受体环丙烷的非对映选择性 [3 + 2] 环加成:四氢吡咯并[1,2-a]quinoxalin-4(5H)-ones 的高效合成
描述了三氟甲磺酸镱催化的喹喔啉酮与供体-受体环丙烷和环丁烷的非对映选择性 [3 + 2] 环加成反应。以高产率(高达 96%)获得一系列四氢吡咯并喹喔啉酮衍生物,并具有优异的非对映选择性(高达 46:1)。其他药用上重要的杂环化合物,如苯并恶嗪酮、异喹喔啉酮和二苯并恶唑衍生物,也适用于所需的环化反应。本方法适用于放大反应。此外,这种环化反应的效用通过密集官能化脯氨酸衍生物的合成得到证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号